Spinal fusion is one of the most common procedures performed in spinal surgery. The increasing complexity and frequency with which this operation is performed has led to a rise in postoperative complications and delayed recovery. Perioperative care of patients with spinal conditions aims to adequately manage pain and accelerate the return to function following surgery. Traditional pharmaceutical interventions, especially opioid utilization, are associated with delayed recovery and significant adverse effects. As such, many studies are now evaluating the benefits and efficacy of non-pharmacological therapies for enhancing recovery after spinal fusion surgery. In this review, the mechanism of acupuncture relative to postoperative pain control and symptom reduction will be discussed. Additionally, this review examines the implications of malnutrition, current preclinical and clinical approaches for improving nutritional status, and various forms of physical rehabilitation that aim to combat postoperative complications and support recovery after spinal fusion surgery.
Acupuncture; Improved Outcomes; Spine Surgery; Recovery
Over the last several decades, the annual number of spinal fusion surgeries has rapidly increased due to the combination of an aging population, technological advances, and expanding indications for fusion [1]. The most prevalent indications are spondylolisthesis, scoliosis, degenerative disc disease, and spinal stenosis; although the volume of surgical intervention for spinal stenosis is declining [2]. Despite its promising results, complications, including non-union, wound infection, and implant failure, are reported in 13.3 to 18.7% of patients undergoing posterior, anterior, or transforaminal lumbar interbody fusion [3]. Multiple factors influence the formation of a solid bony union. Systemic host factors include smoking status, nutritional status, growth hormone levels, inflammatory state, and mechanical stress [4,5]. Surgical site factors include the choice of bone graft material, the number of fusion levels, and biomechanical properties of the fusion construct [6].
The rising number of spinal fusions is associated with a rise in the number of pseudarthroses. Therefore, improving recovery after spinal fusion surgery presents a significant challenge for both patients and surgeons. Interventions such as preoperative education, multimodal analgesia, prevention of postoperative nausea and vomiting, and antimicrobial prophylaxis have been adopted to minimize complications and reduce postoperative pain. Studies have also evaluated the benefits of alternative therapies in spinal fusion. Increasing evidence demonstrates the efficacy of acupuncture alone or as an adjunct to pharmaceutical therapies for pain management after spinal surgery. Adequate pain control in the postoperative period allows for early patient mobilization, which promotes proper fusion [5]. Sufficient and correct loading of the joint prevents matrix reabsorption and pseudoarthrosis [5]. Furthermore, optimizing nutritional status prior to surgery has been shown to prevent postoperative complications and reduce disability after surgery [7].
This review provides an overview of the pathophysiology of healing after spinal fusion surgery and a discussion of the current research on complementary approaches, including acupuncture, nutritional augmentation, and exercise, that can be integrated into rehabilitation programs to improve recovery after spinal fusion surgery.
Spinal fusion involves decompressing the nerve root and immobilizing the vertebral joint to alleviate pain caused by instability and traction upon the joint capsule [8]. While temporary support is achieved by placing an implant in the intervertebral disc space, permanent support requires the formation of a solid osseous union of the adjacent vertebrae and implant.
For fusion to succeed, osteoprogenitor cells must mature into osteoblasts that permeate the fusion matrix (allograft, autograft, or biomatrix) and deposit bone. Graft incorporation follows a similar process as fracture healing and can be divided into five overlapping stages: inflammation, vascularization, osteoinduction, osteoconduction, and remodeling, as shown in Figure 1. [9,10].
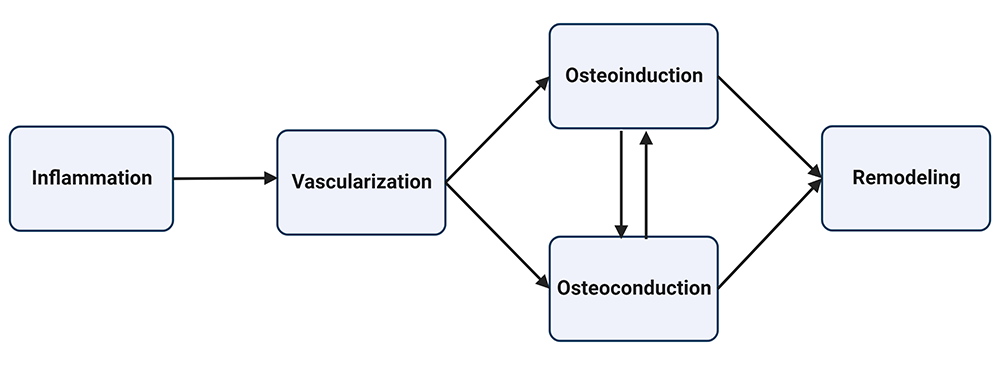
Figure 1: Graft incorporation occurs in five stages. The inflammatory phase begins immediately after surgical intervention, followed by the vascularization phase. After 2-3 weeks, osteoinduction begins, followed by osteoconduction. Osteoinduction and osteoconduction can occur simultaneously as stem cells differentiate into osteoblasts that grow into the fusion matrix. Remodeling occurs during the final stage as osteoblast and osteoclast activity lead to a fully mineralized bone matrix.
The inflammatory cascade is initiated by surgical trauma and comprises the first 14 days of the postoperative period [11]. The infiltration of immune cells, pro-inflammatory cytokines (IL-1, IL-6, CRP, TNF, RANKL), transforming growth factors-β (Bone Morphogenetic Protein-2 (BMP2)), and angiogenic factors is responsible for enhancing extracellular matrix synthesis, angiogenesis, and infection control [10,12]. The inflammatory response peaks on postoperative day 1 after spinal instrumentation surgery, though the body simultaneously mounts an anti-inflammatory response [12]. Nevertheless, there seems to be a necessary level of inflammation present during early bone healing as lower rates of fusion are observed among patients treated with anti-inflammatory medications such as NSAIDs, COX-2 inhibitors, and steroids [10,13,14].
Next is the vascularization phase, where vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and epidermal growth factor (EGF) promote continued vascular proliferation. This provides access for osteoprogenitor cells and nutrients to supply the distal matrix. The initial stages of healing are susceptible to nicotine as it causes vasoconstriction and endothelial damage leading to delayed revascularization of bone grafts, the formation of zones of necrosis, and a significantly elevated pseudoarthrosis rate [15,16].
Osteoinduction begins 2 to 3 weeks after transplantation as stem cells differentiate into osteoblasts and form a callus. This process is largely determined by local host and graft factors, especially BMP-2[5]. Osteoconduction occurs as osteoblasts grow into the fusion matrix and synthesize new bone. The osteoinductive and osteoconductive properties of implant materials are topics of ongoing research aiming to manipulate the bone healing process. The remodeling phase begins as osteoblast activity fills the matrix with new bone while osteoclast activity resorbs the graft. The outcome is a fully loadable reconstructed osseous union of two vertebrae.
Successful fusion is clinically assessed by radiographic findings and patient-reported outcomes. Assuming that pathological motion and reduced mechanical integrity of the spine cause pain and disability, patients achieving a solid osseous union would be expected to have better clinical outcomes than those who do not. On this basis, recent guidelines make a Grade B recommendation that improving radiographical fusion can improve clinical outcomes after lumbar arthrodesis for degenerative disease [17]. However, studies considered in this guideline provide evidence against a correlation between radiographic fusion and clinical outcomes [18-20]. Therefore, clinical suspicion and patient-reported symptoms should be considered independently of radiographs to detect pseudoarthrosis.
In a comprehensive review evaluating outcome measures of treatments for chronic low back pain, Chapman et al. emphasize the utility of outcome measures that prioritize pain, function, and quality of life [21]. Postoperative pain is associated with decreased patient satisfaction, delayed postoperative ambulation, and increased morbidity and mortality [22]. In a recent review of 179 surgical procedures, spinal procedures were associated with a higher level of postoperative pain than other surgical procedures. The second and third highest ranking pain scores on the first postoperative day are lumbar fusion of one to two segments and lumbar fusion of three or more segments, respectively [23].This study concluded that pain treatment after major spinal surgery is insufficient as epidural anesthesia was not used for this population, and mean opioid doses were lower than the patient-controlled analgesia group [23]. These findings suggest that pain is a significant clinical measure requiring greater attention in postoperative patient care.
Pain intensity has implications beyond the immediate postoperative period. In a study of 260 patients undergoing a surgical laminectomy with or without fusion for spinal stenosis, changes in pain intensity at one visit were predictive of improvements in physical function and reductions in disability at the following visit for the first year after surgery [24]. Beyond that, some patients experience persistent postoperative pain syndrome (PPP), also termed failed back surgery syndrome, which comprises chronic back and leg pain that persists despite surgical intervention [25]. Patients experiencing PPP following lumbar surgery report a lower health-related quality of life, decreased function, increased work disability, and higher annual medication costs compared to patients experiencing other common pain syndromes, such as rheumatoid arthritis, osteoarthritis, fibromyalgia, and complex regional pain syndrome [25]. Patients with PPP also have significantly higher healthcare usage, primarily due to a higher average cost of pain medications. They are also twice as likely to have two or more inpatient hospital stays in the first two years after surgery [26]. Therefore, effective pain control in the perioperative period is necessary for improving surgical outcomes.
Overall, successful fusion depends on specific microscopic changes at the cellular level in addition to macroscopic changes such as correct loading of the joint, radiographic changes, and pain reduction. Complementary approaches, including acupuncture, nutritional augmentation, and exercise, can be integrated into rehabilitation programs to improve recovery after spinal fusion surgery.
Acupuncture has been a major therapeutic method in the Far East for thousands of years. Traditional Chinese Medicine, including acupuncture, is based on the concept of a vital force, called Qi, that travels through channels in the body called meridians. When there is an interruption of flow or an imbalance of Qi, disease can occur. Stimulation of acupoints located along the network of meridians is believed to have a therapeutic effect by restoring Yin-Yang balance [27]. Acupuncture research has markedly increased as it gained popularity in the western world and is now widely used for pain management and the treatment of conditions including stroke, nausea, vomiting and mental health management [28].
Mechanisms of Acupuncture Analgesia
To evaluate the role of acupuncture in improving recovery post-spinal fusion surgery, it is important to assess the purported mechanisms by which it causes pain relief. An early and popular theory to explain acupuncture analgesia posits that acupuncture promotes the release of endogenous opioids from lymphocytes, monocytes/macrophages, and granulocytes [29] and increases the opioid receptor affinity and number [30]. Following acupuncture treatment, the expression of opioid peptides, including endomorphin (µ-opioid agonist), dynorphin (κ-opioid agonist), enkephalin and β-endorphin (µ- and δ-opioid agonists), is upregulated. The binding of opioid peptides to their receptors on central neurons induces antinociception [30]. In 2010, Taguchi et al. investigated whether endogenous peripheral opioid receptors participated in electroacupuncture analgesia in a rat model for inflammatory pain. Before undergoing treatment with acupuncture, each rat was injected with a selective or nonselective opioid receptor antagonist. The results demonstrated a dosage-dependent blockade of acupuncture-induced inhibition of mechanical hyperalgesia [31,32]. Taguchi et al. concluded that electroacupuncture activates peripheral µ, κ, and δ receptors to induce analgesia.
Acupuncture is also suggested to act by modulating the inflammatory response through mechanical stimulation of the hypothalamic-pituitary-adrenal (HPA) axis and autonomic pathways [33]. Activation of the HPA axis promotes the release of glucocorticoids from the adrenal glands leading to transactivation of anti-inflammatory cytokines, IL-4 and IL-10, as well as blockade of pro-inflammatory transcription factors, NF-kB (Nuclear factor kappaB) and AP-1(activator protein-1) [33]. Vagal stimulation is achieved at various acupoints and has been found to attenuate serum concentrations of NF-kB, in addition to inflammatory cytokines IL-6 and IL-1b [33]. Specifically, electroacupuncture at the ST36 acupoint in a neuropathic surgery mouse model upregulated serum IgG concentration, promoting neurological recovery and inhibiting spinal glial cell activation [34]. Electroacupuncture (EA) at ST36 also restored the level of vitamin D precursor in this mouse model [35]. Another proposed anti-inflammatory mechanism is acupuncture-induced microtrauma. Local detection of damage by A- and δ- and C- fibers activates the neuro-immune reflex to release vasoactive substances and triggers the local inflammatory-anti-inflammatory response. Adenosine is also released in response to microtrauma and acts on A1 receptors in sensory afferents of ascending nerve tracts to produce antinociceptive properties [27,29]. Acupuncture is suggested to dampen the transmission of noxious input at the spinal level by interfering with the activity of serotonin, norepinephrine, glutamate, substance P γ-aminobutyric acid (GABA), dopamine receptors and various signaling molecules [29].
As illustrated in Figure 2, acupuncture acts through various proposed pathways to produce analgesic and anti-inflammatory effects. Mainly, it relies on the release of endogenous opioids, inflammatory mediators, and local microtrauma.
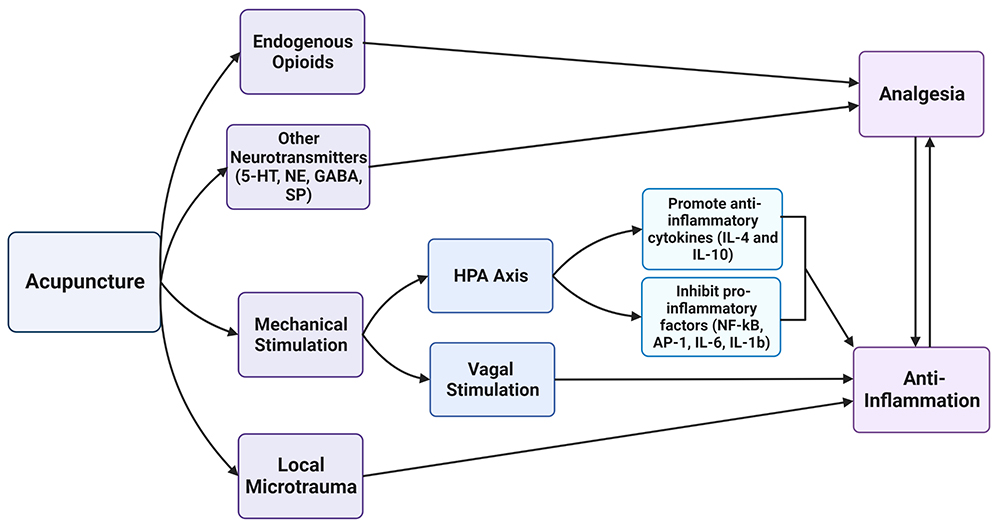
Figure 2: Acupuncture yields direct analgesic effects by acting on endogenous opioid receptors or interfering with the transmission of noxious stimuli by other neurotransmitters. The mechanical stimulation at acupoints acts through intermediate mechanisms at the HPA axis and vagus nerve to indirectly promote anti-inflammatory effects. Local microtrauma from acupuncture needles activates the neuro-immune reflex, which modulates the immune response. A reduction in inflammation can lead to pain relief and vice versa. 5-HT, serotonin; NE, norepinephrine; GABA, γ-aminobutyric acid; SP, substance P; NF-kB, nuclear factor kappaB; AP-1, activator protein-1.
Current research is exploring new targets potentially involved in acupuncture analgesia. In 2021, Wang et al. investigated changes in the level of endocannabinoid receptor 1 (CB1) following electro acupuncture in the incisional pain rate model [36]. Their findings revealed that the expression of CB1 was upregulated after EA stimulation, providing evidence for its role in pain relief [36]. Another study investigated whether EA stimulation protects the motor endplate and enhances recovery in rats with spinal cord injuries [37]. Their findings concluded that EA treatment in rats upregulated the expression of synaptic differentiation marker Tuj-1 while suppressing the inflammatory response [37]. EA also reduced muscular atrophy in rats while limiting the formation of glial scars and recovering motor function [37]. In addition, Zhang et al. demonstrate that EA promotes the growth of new neurons while inhibiting astrocyte activation, favoring overall spinal cord repair. Despite the wide range of proposed mechanisms of acupuncture action, pain reduction and inflammatory modulation remain the main targets. As more studies evaluate the role of acupuncture in modern medicine, it is important to recognize that the physiological activity of acupuncture therapy can vary, as seen with different animal models.
Clinical Applications
Perioperative acupuncture provides clinically significant benefits for patients. It helps to reduce preoperative anxiety, postoperative nausea and vomiting, consumption of anesthetics and analgesics, and hemodynamic instability.
Preoperative care
Preoperative anxiety is linked to sustained anxiety after surgery, increased postoperative sensitivity to pain, suppressed immune system, increased postoperative infection, and prolonged recovery time [38]. In a 2014 meta-analysis of randomized controlled trials (RCTs), Bae et al. demonstrated that acupuncture could decrease preoperative anxiety compared to non-treatment and sham treatment conditions. This relationship held true for all types of acupuncture, including acupuncture needles, acupressure balls or beads, electroacupuncture, auricular acupoints, and body acupoint stimulation. The acupoints used to decrease preoperative anxiety were the third eye (Yin-Tang) and the relaxation auricular point [39]. In a 2021 meta-analysis, Tong et al. also concluded that acupuncture therapy could decrease anxiety assessment scores in patients with preoperative anxiety; however, they reported that the evidence is of moderate to low quality and that further research is necessary to assess the reliability of these results [40]. Acupuncture could also improve a patient’s preoperative condition by stabilizing blood pressure and blood sugar levels and lowering the risk of intraoperative anesthesia, thus reducing the risk of postoperative complications and supporting proper recovery [38].
Intraoperative/Postoperative care
Spinal fusion surgery has been rated as one of the most painful procedures regardless of the number of levels fused [23]. Poorly controlled pain limits patient mobility which may ultimately increase the risk of complications including, deep vein thrombosis, pulmonary embolus, and pneumonia [41]. Successful pain management in the perioperative period can improve surgical outcomes, reduce hospital length of stay (LOS), and decrease the development of chronic pain conditions such as PPP [41]. Opioid analgesics are the most widely used agent for postoperative pain control. Despite being the first-line analgesic therapy in this setting, its overuse is associated with significant adverse effects ranging from nausea, vomiting, and bowel dysfunction to somnolence and respiratory depression. Although multimodal pain management protocols suggesting the addition of NSAIDs, acetaminophen, anticonvulsants, muscle relaxants, and neuraxial blockade demonstrated improved pain management with less reliance on opioids, there remains a concern for adverse effects on bone fusion, liver toxicity, and the central nervous system [41]. Acupuncture provides an additional mode of analgesia with minimal adverse outcomes and similar effects of reduced opioid demands.
A systematic review on the effectiveness of acupuncture and acupuncture-related techniques (EA or transcutaneous electric acupoint stimulation (TEAS)) for treating acute postoperative pain identified thirteen RCTs with a total of 682 patients. All trials had two groups to compare acupuncture to control therapy. Although the location and timing of treatment relative to surgery varied among studies, patients treated with acupuncture or related techniques experienced less pain and used fewer opioid analgesics on the first day after surgery compared to the control group (p<0.001). Further subgroup analysis concluded that conventional acupuncture and TEAS were associated with less postoperative pain on the first day after surgery, while EA had similar effects as the control group. Additionally, TEAS had a greater association with reduced opioid analgesic use compared to other acupuncture techniques and control treatments [42].
More recently, a 2022 systematic review of 12 RCTs with 904 patients assessed the effect of acupuncture combined with patient-controlled analgesia (PCA) on acute postoperative pain and opioid use reduction after back surgery. The results showed that acupuncture with PCA was associated with lower visual analog scale (VAS) scores for pain and less postoperative opiate analgesia compared to sham acupuncture with PCA or PCA alone. Moreover, acupuncture with PCA reduced the incidence of overall PCA-related complications, compared to PCA alone, further demonstrating the benefits of acupuncture in back surgery [43]. Also, in 2022, Chen et al. published a retrospective study evaluating acupuncture as adjuvant therapy for pain control after surgery for degenerative lumbar disease. The study analyzed 96 patients receiving acupuncture, PCA, or routine analgesics in addition to regular acetaminophen/ibuprofen. On postoperative day 6, patients in the acupuncture group had significantly lower VAS scores than the other two groups (p=0.047). However, there was no statistical difference on postoperative days 1 and 2 [44]. In addition, a significantly greater number of patients treated with acupuncture reported complete or much improvement of their back pain after surgery than patients in the PCA or routine analgesics groups [44]. Therefore, these results highlight that as a conservative mode of analgesia, acupuncture may offer similar or better outcomes than pharmacologic treatments with a considerably lower risk of side effects. Another similarly constructed study retrospectively evaluated the effectiveness of acupuncture for treating postoperative pain in patients with degenerative lumbar disease [45]. In this study, acupuncture had a greater acute analgesic effect than standard oral pain relievers, a comparable effect to PCA, and prevented rebound pain during the monitoring period [45]. Subgroup analysis of patients receiving preoperative acupuncture or not revealed that those who received preoperative acupuncture intervention experienced stronger analgesic effects on the third postoperative day and less rebound pain during their recovery [45]. Despite its limitations of a small sample size and the subjective perception of difference, this study suggests the potential overall superiority of acupuncture compared to standard treatment options and introduces how factors such as treatment timing can influence patient outcomes.
While most studies evaluate the role of acupuncture in acute pain reduction after back surgery, very few trials have assessed the effectiveness of acupuncture for non-acute pain after back surgery. Heo et al. conducted a pilot RCT in 2018 on 40 patients with at least 3 weeks of postoperative lower back pain who were allocated to receive either usual care (UC, drug therapy, physiotherapy, and education) alone or UC in conjunction with EA. Both groups received eight treatments over 4 weeks. At 4 and 8 weeks after treatment, outcomes of back pain intensity, back pain-related disability, and quality of life were measured using the VAS score, Oswestry disability index (ODI), and EuroQol Five Dimensions (EQ-5D) questionnaire, respectively. Although there were no statistically significant differences in the VAS score and EQ-5D between the two treatment groups at 8 weeks, there was a statistically significant decrease in ODI after 8 weeks in the EA plus UC group compared to UC alone [46]. Though the findings of this RCT are limited by its nature as a pilot study, the improvement in disability scores is promising and merit further trials to determine the extent of long-term benefits.
Postoperative nausea and vomiting (PONV) present a barrier to early discharge after spine surgery. Since opioid medications contribute to PONV, an emphasis is placed on minimizing their administration and, instead, using non-opioid analgesia to limit these side effects [47]. To help combat the incidence of PONV, acupuncture and acupressure have been investigated as alternatives to current pharmacologic treatments. In a single-blinded, sham-controlled study of 127 patients, patients receiving auricular acupressure from returning to the ward until postoperative day 2 had a significantly reduced severity of PONV during the first 72 hours after surgery [48]. A 2015 Cochrane Systematic Review of 59 RCTs with 7667 patients found that stimulation at the PC6 acupoint was as effective as antiemetic prophylaxis at preventing PONV and reducing the need for rescue antiemetics, compared to sham treatment [49].
As summarized in Table 1, acupuncture and similar techniques can improve postoperative pain and reduce opioid use in the perioperative setting. Several studies demonstrated the ability of acupuncture to achieve greater pain control than other methods without the side effects of opioids, NSAIDs, or PCA. Acupuncture was also found to be safe, with no or minimal recognized adverse effects [50,51].
Table 1: A summary of the clinical benefits of acupuncture therapy in the preoperative and postoperative/intraoperative settings.
|
Preoperative
|
Postoperative/Intraoperative
|
|
Reduces anxiety
Stabilizes blood pressure
Stabilizes blood glucose
|
Reduces acute pain
Decreases opioid demand
Reduces PCA-related complications
Reduces disability
Prevents/reduces PONV
|
In the past 10 years, there appears to be a greater number of studies evaluating the use of acupuncture for the management of postoperative pain after surgery. Due to variations in pain control regimens and limited generalizability of the findings, acupuncture will remain an attractive option for postoperative pain management but requires rigorous controlled, prospective studies to definitively establish safety and superiority over conventional methods.
Preclinical Nutritional Augmentation
Malnutrition has been shown to significantly increase complications, mortality rates, and LOS in patients recovering following lumbar spinal fusion [52]. Malnutrition has also been linked to an increased risk of developing surgical site infections following surgery, and malnutrition has been correlated with patients requiring multiple surgical interventions [53,54]. As a preventative measure for improving postoperative outcomes, nutritional supplementation is recommended for malnourished patients before surgery, especially since it is an independent risk factor for infection and wound complications following spinal fusion [55].
Vitamin D is a secosteroid hormone necessary for calcium and phosphate absorption. In the musculoskeletal system, the activity of vitamin D is responsible for bone mineralization and is positively associated with bone mineral density. Prolonged and severe vitamin D deficiency leads to rickets in children and osteomalacia in adults [56]. Vitamin D deficiency also exacerbates osteopenia, osteoporosis, and fractures in adults and is a risk factor for cancers, autoimmune diseases, hypertension, and infectious disease [57]. Although vitamin D has been extensively studied for its role in overall bone health, there is conflicting data regarding vitamin D supplementation during bone repair and post-traumatic bone turnover [58]. In rat models undergoing posterolateral inter-transverse process spinal fusion, those fed a hyper-vitamin D diet preoperatively had improved quantitative measures of femoral biomechanics and geometry, including femoral strength, stiffness, density, and cortical thickness. Although the spinal fusion rate and stiffness did not correlate to the above changes in femoral characteristics, this study describes a significant positive relationship between spinal stiffness to femur max load.59 Interestingly, this study also observed a decrease in serum vitamin D levels during fusion healing. Therefore, they concluded that dietary vitamin D above normal level improves bone health in a rat posterolateral spinal fusion model [59]. An overview of the benefits of Vitamin D on bone health is shown in Figure 3.
Vitamin E is an antioxidant stress agent that affects the bone remodeling process and has shown preventative effects on bone loss in osteopenic women through anti-resorptive activity [60]. The Vitamin E isomer, a-tocopherol, has been found to prevent osteoporosis and improve bone fracture healing by purportedly increasing the activity of antioxidant enzymes. These enzymes neutralize excess free radicals released during the early phase of fracture healing in osteoporotic bone [61,62].
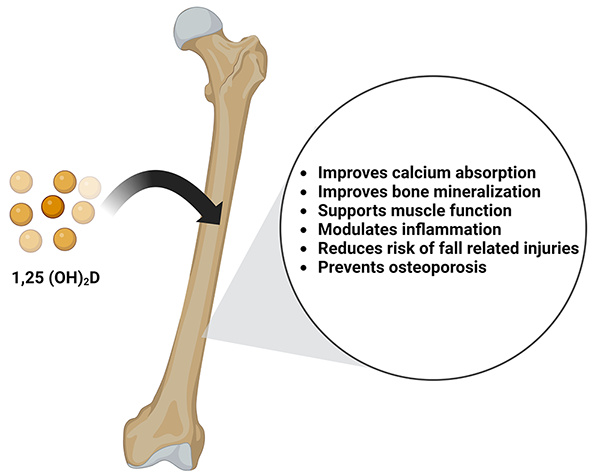
Figure 3: Vitamin D is converted into its active form of 1,25- dihydroxycholecalciferol (1,25(OH)2D). It benefits bone health by improving calcium absorption, promoting bone mineralization, supporting muscle function, modulating inflammation, reducing the risk of fall-related injuries, and prevents osteoporosis.
As mentioned in the above discussion of bone healing, osteogenic cells need to travel to the fracture or fusion site to begin remodeling and repair. Stimulation of osteoblast migration at this stage could be a promising strategy for enhancing osteoanabolic functions. δ-tocotrienol is a form of Vitamin E that stimulates the migration of both MC3T3-E1 osteoblast-like cells and primary human bone marrow mesenchymal stem cells (BMSC). The δ-tocotrienol-promoted migration of MC3T3-E1 cells depends on Akt phosphorylation and activation of the Wnt/β-catenin signaling pathway[63].In the study by Casati et al., treatment of BMSCs with δ-tocotrienol increased β-catenin transcriptional activity and, when inhibited, reduced wound healing activity of δ-tocotrienol and MC3T3-E1 cells. The δ-tocotrienol treatment also increased the expression of β-catenin target genes, osteocalcin, and BMP-2, involved in osteoblastic differentiation and migration [63,64].
Red yeast rice is produced by the fermentation of mycelia of Monascus purpureus and has shown potential as an agent for bone formation [65]. Red yeast rice promotes the proliferation of osteoblasts and increases the expression of alkaline phosphatase (ALP), a glycoprotein related to osteoblastic function. It is suspected to act via the BMP-2/Smad signaling pathway by increasing the BMP content of mesenchymal stem cells, inducing their differentiation into osteoblasts. Additionally, red yeast rice-induced improvement of bone mineral density may be related to the promotion of bone matrix mineralization by osteoblasts [66].
Clinical Nutrition Augmentation
Malnutrition in patients undergoing elective spinal fusion surgery could prolong inflammation, impede wound healing, and increase the risk of surgical site infection [7,67]. Considering the morbidity and mortality associated with these complications, nutritional status is critical following spinal fusion. To assess the nutritional status, serologic parameters such as total lymphocyte count, albumin level, prealbumin level, and transferrin levels can be used. Preoperative hypoalbuminemia has been shown to increase morbidity, readmissions, reoperations, and mortality following spine surgery [7,68,69], preoperative albumin levels of less than 20mg/dl was shown as a predictor for developing postoperative infection [70]. and future research is needed to help elucidate the predictive value of preoperative transferrin levels on postoperative complications [71].
Preoperative nutrition assessment and optimization of nutritional parameters, including blood glucose, serum albumin, and body weight, may reduce the risk of perioperative complications [72]. Poor wound healing resulting from diabetes showed significantly higher postoperative infection rates, a 5-fold increase compared to preoperative nondiabetic patients [7]. For obese patients, spinal fusion operative times are longer, occur with greater blood loss, and have more postoperative complications [73-76]. Hypoalbuminemia can present in obese patients due to inadequate protein intake despite excessive calorie consumption, serving as a significant risk factor for delayed wound healing. Additionally, increased distance from the skin to the lamina and thickness of subcutaneous tissue significantly raised surgical site infection rates [77].
Diagnosing malnutrition prior to surgery is vital as it influences postoperative patient outcomes.78 For instance, determining albumin, transferrin, and lymphocyte counts prior to spinal fusion can identify potential adverse events [78]. Low levels of these parameters have an increased risk of surgical site infections, LOS, postoperative complications, and mortality [55,78] Interventions before surgery can be taken to reduce the likelihood of complications. One study found that a powder containing protein, carbohydrates, and other nutritional elements was linked to a shorter LOS, improved management of electrolyte imbalances, and higher albumin levels following surgery [67]. Additionally, oral nutritional supplements are associated with improved outcomes compared to the standard clinical care [79].
Preoperative consumption of more than 2 units of alcohol increases the risk of adverse events following spinal surgery [80]. Deep vein thrombosis, delirium, bleeding, cardiopulmonary complications, and infections are some complications associated with alcohol consumption following spinal surgery [78]. Some studies investigated the efficacy of alcohol cessation interventions 1-2 months prior to surgery and found that it reduces the incidence of postoperative complications but not mortality [81]. Interventions focusing on behavior changes, vitamins, benzodiazepines, and disulfiram are also suggested to reduce the risks following surgery [78].
About one-third of patients undergoing elective surgery have preoperative anemia associated with higher complication and readmission rates. Preoperative anemia in patients undergoing elective spine surgery significantly increases the risk of transfusions, LOS, and poorer outcomes at 30 days [82,83]. Therefore, screening for and treating anemia before and after elective spine surgery can be expected to improve outcomes. A 2017 international consensus statement recommends managing iron deficiency with oral iron in the 6-8 weeks before surgery [84]. Although evidence supports interventions such as oral iron supplements, iron infusions, or erythropoietin for anemic patients undergoing spine surgery, there remains a debate over the target hemoglobin needed for meaningful improvements in outcomes [78]. Therefore, the current guidelines recommend using minimally invasive techniques to minimize blood loss and consulting hematology for further guidance [78].
In one RCT, researchers investigated the impacts of a new multimodal nutritional management (MNM) protocol consisting of nutritional powders for patients following spinal fusion [67]. The study found that their specific powder regimen reduced the total amount of albumin infused and increased the postoperative albumin level [67]. Additionally, the incidence of wound drainage declined, accelerating wound healing and reducing the risk of infection [67]. The MNM protocol decreased the occurrence of hypokalemia, hyponatremia, and hypocalcemia [67]. Overall, the study found that a nutritional intervention reduced the risk of electrolyte disorders, wound drainage, and LOS while remaining safe and effective for patients [67].
Vitamin D deficiency is associated with muscle weakness, inflammation, diminished citrate synthase activity and calcium binding in bone, and reduced content of PGC-1α in skeletal muscle of patients with low back pain. There also is an association between vitamin D deficiency and spine morbidities [85]. Vitamin D supplementation has also been shown to further improve the effects of early rehabilitation following spine fusion by reducing muscle atrophy of the muscles responsible for lumbar spine stabilization [86]. In one study, the prevalence of vitamin D deficiency amongst patients requiring decompression surgery was 76.5% [87]. Maintaining 25-hydroxy vitamin D levels in the serum pre and postoperatively was necessary to enhance the long-term functional outcomes of the spine [87]. Adequate levels of vitamin D have been shown to yield stiffer spinal fusions, bone volume, and density compared to inadequate levels [88].
Protein supplementation reduces post-fracture bone loss, increases muscle strength, and reduces medical complications and rehabilitation hospital stay [89]. Maintaining adequate protein intake improves the fusion of vertebrae by various mechanisms, such as optimizing IGF-1 levels, increasing intestinal calcium absorption, transporting phosphorus, providing structural bone matrix, and improving muscle strength [90]. Atrophy of the paraspinal muscles can cause weakness following posterior spinal fusion surgery, leading to pain and dysfunction postoperatively. One study assessed the effect of protein supplementation for four weeks and found that it led to the significant enlargement of the cross-sectional area of multifidus and psoas muscles compared to the placebo group. In addition, less atrophy was observed in the erector spinae and quadratus lumborum muscles, and protein consumption was negatively correlated with pain [91].
The approach to achieving successful spinal fusion is multifaceted. As a result, the healthcare team should optimize surgical outcomes through personalized preoperative, intraoperative, and postoperative interventions based on the individual patient status and diagnosis. Diet modifications are a practical and effective way of improving postoperative spinal fusion complications, and further research on this topic could be promising.
Rehabilitation programs tailored to specific exercises are often needed in patients following spinal fusion surgery [92]. These programs include generalized cardiovascular exercises, motor control, and stability training, soft-tissue mobilization, neural mobilization, and patient education, each with various outcomes and benefits [93].
After surgery, patients often experience chronic lower back pain, so therapies that improve health without increasing pain are recommended [92]. For instance, cardiovascular exercise that improves aerobic functions has beneficial health impacts without negatively affecting lower back pain postoperatively [94]. By focusing on cardiovascular exercises, such as walking, patients can decrease their pain while increasing function, both preoperatively and postoperatively [95,96]. Preoperatively, cardiovascular exercise has been shown to decrease the LOS following surgery and enhance overall health [93]. In patients with an impaired walking tolerance, using recumbent bikes increases cardiovascular health before the procedure [93]. Early interventions that highlight the importance of mobility have reduced the LOS postoperatively, the incidence of adverse events, and back pain[93].
Studies have demonstrated that lumbar extensor function and density are adversely affected following spinal fusion [97,98]. Muscle atrophy following surgery is a concern; therefore, emphasis is placed on strengthening trunk extensors [99,100]. Proper neuromuscular reeducation ensures efficient and effective muscle movement to promote muscle healing and to adapt to the new restricted range of motions [93]. One study found that core strengthening exercises are also beneficial to the spine postoperatively by decreasing ODI scores [100]. Certain loading exercises (lifting, bending, transfers) have improved neuromuscular reeducation events like timing for muscle activation [93]. Increasing core strength through spine control exercises can be achieved through postoperative rehabilitation and has been shown to decrease disability scores [93].
Soft-tissue mobilization is frequently employed following spinal fusion surgery to reduce pain and inflammation by reestablishing lymphatic drainage, relaxing muscles, and improving blood circulation [93]. Additionally, massage therapy relieves patient anxiety and tension [101,102]. After spinal fusion, it is important to maximize flexibility and reduce pain [103]. In a study investigating the efficacy of massage therapy following lumbar fusion, Keller found that pain levels decreased following the intervention [93]. However, the intervention only demonstrated short-term results as patients reported pain recurrence one week later, before the subsequent massage therapy. Despite the lack of long-term pain reduction, massage therapy remains a beneficial supplementary mode of pain management without adverse effects [103]. Additionally, soft-tissue mobilization may be particularly useful in patients with anxiety or distress during recovery or in those with swelling and pain at the incision site [93].
Pain after spinal fusion can be attributed to nerve root attachment to the scar tissue around the decompressed sac [93]. Multiple studies have explored whether neural mobilization should be used to prevent nerve root adherence and improve overall outcomes. Neural mobilization includes using both passive movements and active exercises to increase the mobility of the lumbosacral nerve roots[104]. However, the efficacy of neural mobilization has been disputed. In one study, researchers compared standard rehabilitation techniques to standard care plus neural mobilization for 12 months[104]. They investigated whether pain improved with this technique and found no additional benefits to neural mobilization compared to the standard care group [104]. Another study also found that standard care plus neural mobilization yields similar pain improvement and disability levels as standard care alone [105]. However, neural mobilization techniques are deemed safe for patients after spinal fusion and could still decrease nerve adherence to scar tissue and improve range of motion.
Patient education is crucial in rehabilitation efforts, as it lowers anxiety and improves overall patient outcomes and satisfaction. Providers should focus on building rapport with the patient preoperatively and establishing realistic expectations[93]. Improving patient education and understanding has been shown to decrease complications and LOS [106]. This allows patients to begin early interventions, such as cardiovascular exercises before surgery [93].
Although physical exercises are associated with various benefits, a 2022 systematic review of eight studies found that cognitive therapy during physical rehabilitation yields more positive outcomes compared to exercise alone [107]. The relationship can be best explained by the fear-avoidance model in which physical and psychological symptoms interact and affect one another. Catastrophizing and kinesiophobia are believed to increase pain and decrease the physical function of patients [107]. A 2013 RCT of 130 patients undergoing lumbar fusion found that cognitive therapy was superior to physical rehabilitation alone in managing kinesiophobia and catastrophizing [92]. Therapy allowed patients to reduce catastrophic thoughts and address fear-avoidance beliefs, resulting in successful improvements in quality of life following spinal fusion surgery [92]. Use of cognitive therapy is recommended by seeking out qualified personnel who are trained in managing chronic pain and spinal disorders [92]. Another longitudinal study published in 2022 notes that patients undergoing spinal fusion often report postoperative pain and poor walking outcomes up to 6 months after the surgery [108]. Factors contributing to poor outcomes included preoperative leg weakness and an elevated BMI [108]. Additionally, patients who reported symptoms of depression also had poorer outcomes when compared to those without [108]. Understanding factors which influence postoperative outcomes is crucial in developing the postoperative management of patients undergoing spinal fusion surgeries.
One study found that exercise positively impacts disability and pain management 6 months following the procedure but remains unclear about long-term benefits [109]. Therefore, an approach that combines cognitive therapy and rehabilitation can improve overall symptoms of pain, quality of life, and satisfaction, especially because a combined approach addresses the role of mental health in postoperative outcomes. In addition to patient education, interventions such as cardiovascular exercise, stability training, and soft-tissue mobilization have yielded positive outcomes in various studies and should be considered for the perioperative management of patients undergoing spinal fusion surgery.
The attainment of proper fusion is complex and influenced by a multitude of clinical factors. There is increasing evidence supporting the use of acupuncture therapy for managing preoperative anxiety, postoperative pain, and postoperative nausea and vomiting, though clinical trials with larger sample sizes and longer follow-ups are necessary to guide further clinical recommendations and its adoption in multimodal analgesia. Other supportive measures for improved recovery after spinal fusion are nutritional interventions, optimization of nutrition parameters, cognitive therapy, physical mobility, and strength training. Although no evidence is available for the effectiveness of combination therapies, the low side effect profile and relative safety of these alternative interventions allow tailored treatment regimens based on individual patient needs.
- Rajaee, SS., Bae, HW., Kanim, LE., Delamarter RB (2012) Spinal Fusion in the United States: Analysis of Trends from 1998 to 2008. Spine (Phila Pa 1976) 37: 67-76. [Crossref].
- Martin, BI., Mirza, SK., Spina, N., Spiker, WR., Lawrence, B., et al. (2019) Trends in Lumbar Fusion Procedure Rates and Associated Hospital Costs for Degenerative Spinal Diseases in the United States, 2004 to 2015. Spine (Phila Pa 1976) 44: 369-376. [Crossref].
- Lenz, M., Mohamud, K., Bredow, J., Oikonomidis, S., Eysel, P., et al. (2022) Comparison of Different Approaches in Lumbosacral Spinal Fusion Surgery: A Systematic Review and Meta-Analysis. Asian Spine J. 16: 141-149. [Crossref].
- Johnson, KG., Alsoof, D., McDonald, CL., Berreta, RS., Cohen, EM., et al. (2022) Malnutrition, Body Mass Index, and Associated Risk of Complications After Posterior Lumbar Spine Fusion: A 3:1 Matched Cohort Analysis. World Neurosurg 163: 89-97. [Crossref].
- Pilitsis, JG., Lucas, DR., Rengachary, SS Bone Healing and Spinal Fusion (2002). Neurosurg Focus 13: 1. [Crossref].
- Cruz, A., Ropper, AE., Xu, DS et al. (2021) Failure in Lumbar Spinal Fusion and Current Management Modalities. Semin Plast Surg. 35: 54-62. [Crossref].
- Adogwa, O., Martin, JR., Huang K et al. (2014) Preoperative Serum Albumin Level as a Predictor of Postoperative Complication After Spine Fusion. Spine (Phila Pa 1976) 39: 1513-1519. [Crossref].
- CLOWARD RB (1953) The Treatment of Ruptured Lumbar Intervertebral Discs by Vertebral Body Fusion. i. Indications, Operative Technique, After Care. J Neurosurg 10: 154-68. [Crossref].
- Cheng, I., Oshtory, R., Wildstein MS (2007) The Role of Osteobiologics in Spinal Deformity. Neurosurg Clin N Am 18: 393-401. [Crossref].
- Claes, L., Recknagel, S., Ignatius A (2012) Fracture Healing Under Healthy And Inflammatory Conditions. Nat Rev Rheumatol 8: 133-43. [Crossref].
- Boden, SD., Sumner DR (1995) Biologic Factors Affecting Spinal Fusion And Bone Regeneration. Spine (Phila Pa 1976) 20: 102-112. [Crossref].
- Takahashi, J., Ebara, S., Kamimura M et al. (2002) Pro-Inflammatory and Anti-Inflammatory Cytokine Increases After Spinal Instrumentation Surgery. J Spinal Disord Tech 15: 294-300. [Crossref].
- Dahners, LE., Mullis BH (2004) Effects of Nonsteroidal Anti-Inflammatory Drugs on Bone Formation and Soft-Tissue Healing. J Am Acad Orthop Surg 12: 139-143. [Crossref].
- Sawin, PD., Dickman, CA., Crawford, NR., Melton, MS., Bichard, WD.,et al. (2001) The Effects of Dexamethasone on Bone Fusion in an Experimental Model of Posterolateral Lumbar Spinal Arthrodesis. J Neurosurg 94: 76-81. [Crossref].
- Daftari, TK., Whitesides, TE., Heller, JG., Goodrich, AC., McCarey, BE., et al. (1994)| Nicotine on the Revascularization of Bone Graft. An Experimental Study in Rabbits. Spine (Phila Pa 1976). 19: 904-911. [Crossref].
- Silcox, DH., Daftari, T., Boden, SD., Schimandle, JH., Hutton WC et al. (1995) The Effect of Nicotine on Spinal Fusion. Spine (Phila Pa 1976).20: 1549-1553. [Crossref].
- Dhall, SS., Choudhri, TF., Eck JC et al.(2014) Guideline Update for the Performance of Fusion Procedures for Degenerative Disease of the Lumbar Spine. Part 5: Correlation Between Radiographic Outcome and Function. J Neurosurg Spine 21: 31-36. [Crossref].
- Epstein NE (2008) An Analysis of Noninstrumented Posterolateral Lumbar Fusions Performed in Predominantly Geriatric Patients Using Lamina Autograft and Beta Tricalcium Phosphate. Spine J. 8: 882-887. [Crossref].
- Penta, M., Fraser RD (1997) Anterior Lumbar Interbody Fusion. A Minimum 10-year Follow-up. Spine (Phila Pa 1976) 22: 2429-2434. [Crossref].
- Thalgott, JS., Fogarty, ME., Giuffre, JM., Christenson, SD., Epstein AK (2009) A Prospective, Randomized, Blinded, Single-Site Study To Evaluate The Clinical and Radiographic Differences Between Frozen and Freeze-Dried Allograft When Used as Part of A Circumferential Anterior Lumbar Interbody Fusion Procedure. Spine (Phila Pa 1976). 34: 1251-1256. [Crossref].
- Chapman, JR., Norvell, DC., Hermsmeyer JT et al. (2011) Evaluating Common Outcomes for Measuring Treatment Success for Chronic Low Back Pain. Spine (Phila Pa 1976) 36: 54-68. [Crossref].
- Kehlet, H., Jensen, TS., Woolf CJ (2006) Persistent Postsurgical Pain: Risk Factors And Prevention. Lancet 367: 1618-1625. [Crossref].
- Gerbershagen, HJ., Aduckathil, S., van Wijck, AJ., Peelen, LM., Kalkman, CJ., et al. (2013) Pain Intensity on The First Day After Surgery: A Prospective Cohort Study Comparing 179 Surgical Procedures. Anesthesiology118: 934-944. [Crossref].
- Skolasky, RL., Wegener, ST., Maggard, AM., Riley LH (2014) The Impact of Reduction of Pain After Lumbar Spine Surgery: The Relationship Between Changes in Pain and Physical Function and Disability. Spine (Phila Pa 1976) 39: 1426-1432. [Crossref].
- Thomson S (2013) Failed back surgery syndrome - definition, epidemiology and demographics. Br J Pain. 7: 56-59. [Crossref].
- Weir, S., Samnaliev, M., KuoTC et al. (2017) The Incidence and Healthcare Costs of Persistent Postoperative Pain Following Lumbar Spine Surgery in The Uk: A Cohort Study Using the Clinical Practice Research Datalink (Cprd) and Hospital Episode Statistics (HES). BMJ Open. 7. [Crossref].
- Zhao ZQ (2008) Neural Mechanism Underlying Acupuncture Analgesia. Prog Neurobiol 85: 355-375. [Crossref].
- Wang, H., Yang, G., Wang, S., Zheng, X., Zhang W et al.(2018) The Most Commonly Treated Acupuncture Indications in the United States: A Cross-Sectional Study. Am J Chin Med 1-33. [Crossref].
- Zhang, R., Lao, L., Ren, K., Berman BM (2014) Mechanisms of Acupuncture-Electroacupuncture on Persistent Pain. Anesthesiology 120: 482-503. [Crossref].
- Gao, P., Gao, XI., Fu, T., Xu, D., Wen Q (2015) Acupuncture: Emerging Evidence for Its Use as an Analgesic (Review). Exp Ther Med 9: 1577-1581. [Crossref].
- Taguchi, R., Taguchi, T., Kitakoji H( 2019) Involvement of Peripheral Opioid Receptors in Electroacupuncture Analgesia for Carrageenan-Induced Hyperalgesia. Brain Res. 1355: 97-103. [Crossref].
- Sekido, R., Ishimaru, K., Sakita M (2003) Differences of Electroacupuncture-Induced Analgesic Effect in Normal and Inflammatory Conditions in Rats. Am J Chin Med. 31: 955-965. [Crossref].
- Zhang, L., Yuan, H., Li, J., Li H (2019) Effect of Acupuncture Therapies Combined With Usual Medical Care on Knee Osteoarthritis. J Tradit Chin Med. 39: 103-110. [Crossref].
- Oh, JE., Kim SN (2021) Anti-Inflammatory Effects of Acupuncture at ST36 Point: A Literature Review in Animal Studies. Front Immunol 12. [Crossref].
- Sung, HJ, Kim, YS, Kim IS et al. (2004) Proteomic Analysis of Differential Protein Expression in Neuropathic Pain and Electroacupuncture Treatment Models. Proteomics 4: 2805-2813. [Crossref].
- Wang, J., Zhang, J., Gao, Y., Chen, Y., Duanmu C et al. (2021) Electroacupuncture Alleviates Hyperalgesia by Regulating CB1 Receptor of Spinal Cord in Incisional Neck Pain Rats. Evid Based Complement Alternat Med. [Crossref].
- Zhang, X., Xu, H., Zhu L et al. (2022) Thoracic Jia-Ji Electro-Acupuncture Mitigates Low Skeletal Muscle Atrophy And Improves Motor Function Recovery Following Thoracic Spinal Cord Injury In Rats. Am J Transl Res.14: 8103-8116. [Crossref].
- Yuan, W., Wang Q (2019) Perioperative Acupuncture Medicine: A Novel Concept Instead of Acupuncture Anesthesia. Chin Med J (Engl) 132: 707-715. [Crossref].
- Bae, H., Min, BI., Cho S (2014) Efficacy 0f AcupunctureiIn Reducing Preoperative Anxiety: A Meta-Analysis. Evid Based Complement Alternat Med. [Crossref].
- Tong, QY., Liu, R., Zhang, K., Gao, Y., Cui, GW., et al. (2020) Can Acupuncture Therapy Reduce Preoperative Anxiety? A Systematic Review and Meta-Analysis. J Integr Med. 19: 20-28. [Crossref].
- Devin, CJ., McGirt MJ (2015) Best Evidence in Multimodal Pain Management in Spine Surgery and Means of Assessing Postoperative Pain and Functional Outcomes. J Clin Neurosci 22: 930-938. [Crossref].
- Wu, MS., Chen, KH., Chen IF et al. (2016) The Efficacy of Acupuncture in Post-Operative Pain Management: A Systematic Review and Meta-Analysis. PLoS One. [Crossref].
- Deng, D., Xu ,F., Wang Y et al. (2022) Efficacy of Acupuncture Combined with Patient-Controlled Analgesia in the Treatment of Acute Pain after Back Surgery: A Meta-Analysis. Pain Research and Management.
- Chen, BA., Deng, WC., Chen MY (2022)Acupuncture for Pain Control After Degenerative Lumbar Spine Surgery. Eur J Med Res.27:167. [Crossref].
- Chao, YL., Rau, YA., Shiue HS et al. (2022) Using a Consensus Acupoints Regimen to Explore The Relationship Between Acupuncture Sensation And Lumbar Spinal Postoperative Analgesia: A Retrospective Analysis of Prospective Clinical Cooperation. J Integr Med. 20: 329-337. [Crossref].
- Heo, I., Hwang, MS., Hwang EH et al. (2018) Electroacupuncture as a Complement to Usual Care for Patients with Non-Acute Low Back Pain After Back Surgery: A Pilot Randomised Controlled Trial. BMJ Open. 8. [Crossref].
- Basques, BA., Ferguson, .J, Kunze, KN., Phillips FM (2019) Lumbar spinal fusion in the outpatient setting: an update on management, surgical approaches and planning. J Spine Surg. 5: 174-180. [Crossref].
- Chung, YC., Chien, HC., Chen, HH., Yeh ML (2014) Acupoint stimulation to improve analgesia quality for lumbar spine surgical patients. Pain Manag Nurs. 15: 738-47. [Crossref].
- Lee, A., Chan, SK., Fan LT (2015) Stimulation of the Wrist Acupuncture Point Pc6 For Preventing Postoperative Nausea And Vomiting. Cochrane Database Syst Rev. [Crossref].
- Zhang, XC., Chen, H., Xu, WT., Song, YY., Gu, YH,, et al. (2019) Acupuncture Therapy for Fibromyalgia: A Systematic Review and Meta-Analysis of Randomized Controlled .Trials. J Pain Res 12:527-542. [Crossref].
- Naguit, N., Laeeq, S., Jakkoju R et al. (2022) Is Acupuncture Safe and Effective Treatment for Migraine? A Systematic Review of Randomized Controlled Trials. Cureus. [Crossref].
- Puvanesarajah, V., Jain, A., Kebaish K et al. (2017) Poor Nutrition Status and Lumbar Spine Fusion Surgery in the Elderly: Readmissions, Complications, and Mortality. Spine (Phila Pa 1976) 42: 979-983. [Crossref].
- Tsantes, AG., Papadopoulos, DV., Lytras T et al.(2020) Association of Malnutrition with Surgical Site Infection Following Spinal Surgery: Systematic Review and Meta-Analysis. J Hosp Infect 104: 111-119. [Crossref].
- Karls, CA., Duey-Holtz, A., Lampone OA, et al. (2021) Prevalence of malnutrition and its associated outcomes in pediatric patients with scoliosis undergoing elective posterior spinal fusion or spine growth modulation - a retrospective review. Stud Health Technol Inform. 280: 235-240. [Crossref].
- Bohl, DD., Shen, MR., Mayo BC, et al. (2016) Malnutrition Predicts Infectious and Wound Complications Following Posterior Lumbar Spinal Fusion. Spine (Phila Pa 1976). 41: 1693-1699. [Crossref].
- Laird, E., Ward, M., McSorley, E., Strain, JJ., Wallace J (2010) Vitamin D and bone health: potential mechanisms. Nutrients. 2: 693-724. [Crossref].
- Holick, MF., Chen TC (2008) Vitamin D Deficiency: A Worldwide Problem With Health Consequences Am J Clin Nutr 87: 1080S-1086S. [Crossref].
- Fischer, V., Haffner-Luntze,r M., Amling, M., Ignatius A (2018) Calcium and vitamin D in bone fracture healing and post-traumatic bone turnover. Eur Cell Mater 35:365-385. [Crossref].
- Bhamb, N., Kanim, L., Maldonado, R., Svet, M., Metzger M (2018) Effect of Modulating Dietary Vitamin D on The General Bone Health of Rats During Posterolateral Spinal Fusion. J Orthop Res 36: 1435-1443. [Crossref].
- Vallibhakara, SA., Nakpalat, K., Sophonsritsuk, A., Tantitham, C., Vallibhakara O (2021) Effect of Vitamin E Supplement on Bone Turnover Markers in Postmenopausal Osteopenic Women: A Double-Blind, Randomized, Placebo-Controlled Trial. Nutrient 13. [Crossref].
- Shuid, AN., Mohamad, S., Muhammad N et al. (2011) Effects of Α-Tocopherol on The Early Phase of Osteoporotic Fracture Healing. J Orthop Res 29: 1732-1738. [Crossref].
- Mohamad, S., Shuid, AN., Mohamed N et al. (2012) The Effects of Alpha-Tocopherol Supplementation on Fracture Healing in A Postmenopausal Osteoporotic Rat Model. Clinics (Sao Paulo) 67: 1077-1085. [Crossref].
- Casati, L., Pagani, F., Maggi, R., Ferrucci, F., Sibilia V (2022) Food for Bone: Evidence for a Role for Delta-Tocotrienol in the Physiological Control of Osteoblast Migration. Int J Mol Sci 21. [Crossref].
- Rossini, M., Gatti, D., Adami S (2013) Involvement of WNT/β-catenin signaling in the treatment of osteoporosis. Calcif Tissue Int 93: 121-32. [Crossref].
- Liu, J., Zhang, J., Shi, Y., Grimsgaard, S., Alraek,T., et al. (2006) Chinese Red Yeast Rice (Monascus Purpureus) for Primary Hyperlipidemia: A Meta-Analysis of Randomized Controlled Trials. Chin Med 1:4. [Crossref].
- Wu, B., Huang, JF., He, BJ., Huang, CW., Lu JH (2020) Promotion of Bone Formation by Red Yeast Rice in Experimental Animals: A Systematic Review and Meta-Analysis. Biomed Res Int. [Crossref].
- Xu, B., Xu, WX., Lao, YJ., Ding, WG., Lu D (2019) Multimodal Nutritional Management in Primary Lumbar Spine Surgery: A Randomized Controlled Trial. Spine (Phila Pa 1976). 44: 967-974. [Crossref].
- Camino-Willhuber, G., Franklin, A., Rosecrance K et al. (2022) Preoperative Hypoalbuminemia and Dialysis Increase Morbidity/Mortality After Spine Surgery for Primary Pyogenic Spinal Infections (ACS-NSQIP STUDY). Surg Neurol Int 13: 193. [Crossref].
- Camino-Willhuber, G., Oyadomari, S., Ochoa J et al.(2022) The Impact of Stratified Hypoalbuminemia and Dialysis on Morbidity/Mortality After Posterior Spinal Fusion Surgery: AN ACS-NSQIP Study. Surg Neurol Int 13: 359. [Crossref].
- Salvetti, DJ., Tempel, ZJ., Gandhoke GS et al. (2015) Preoperative Prealbumin Level As A Risk Factor For Surgical Site Infection Following Elective Spine Surgery. Surg Neurol Int 6: S500-503. [Crossref].
- Mbagwu, C., Sloan, M., Neuwirth AL et al. (2020) Preoperative Albumin, Transferrin, and Total Lymphocyte Count as Risk Markers for Postoperative Complications After Total Joint Arthroplasty: A Systematic Review. J Am Acad Orthop Surg Glob Res Rev 4. [Crossref].
- Cross, MB., Yi, PH., Thomas, CF., Garcia, J., Della Valle CJ (2014) Evaluation Of Malnutrition In Orthopaedic Surgery. J Am Acad Orthop Surg 22: 193-199. [Crossref].
- Vaidya, R., Carp, J., Bartol, S., Ouellette, N., Lee, S., et al. (2009) Lumbar spine fusion in obese and morbidly obese patients. Spine (Phila Pa 1976) 34: 495-500. [Crossref].
- Djurasovic, M., Bratcher, KR., Glassman, SD., Dimar, JR., Carreon LY(2008) The Effect of Obesity on Clinical Outcomes After Lumbar Fusion. Spine (Phila Pa 1976) 33: 1789-1792. [Crossref].
- De la Garza-Ramos, R., Bydon, M., Abt NB et al.(2015) The Impact of Obesity on Short- and Long-Term Outcomes After Lumbar Fusion. Spine (Phila Pa 1976) 40: 56-61. [Crossref].
- Sorimachi, Y., Neva, MH., Vihtonen K et al.(2016) Effect of Obesity and Being Overweight on Disability and Pain After Lumbar Fusion: An Analysis of 805 Patients. Spine (Phila Pa 1976)41: 772-777. [Crossref].
- Aleem, IS., Tan, LA., Nassr, A., Riew KD (2020) Surgical Site Infection Prevention Following Spine Surgery. Global Spine J 10: 92S-98S. [Crossref].
- Debono, B., Wainwright, TW., Wang MY et al. (2021) Consensus Statement For Perioperative Care In Lumbar Spinal Fusion: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. Spine J. 21:729-752. [Crossref].
- Nieuwenhuizen, WF., Weenen, H., Rigby, P., Hetherington MM (2010) Older Adults And Patients In Need Of Nutritional Support: Review Of Current Treatment Options And Factors Influencing Nutritional Intake. Clin Nutr 29:160-169. [Crossref].
- Meng, F., Cao, J., Meng X (2015) Risk Factors For Surgical Site Infections Following Spinal Surgery. J Clin Neurosci. 22: 1862-1866. [Crossref].
- Shabanzadeh DM, Sørensen LT. Alcohol Consumption Increases Post-Operative Infection but Not Mortality: A Systematic Review and Meta-Analysis. Surg Infect (Larchmt). Dec 2015;16(6):657-68. [Crossref].
- Seicean, A., Seicean, S., Alan N et al. (2013) Preoperative Anemia And Perioperative Outcomes In Patients Who Undergo Elective Spine Surgery. Spine (Phila Pa 1976) 38: 1331-1341. [Crossref].
- Lange, N., Stadtmüller, T., Scheibel S et al.(2022) Analysis Of Risk Factors For Perioperative Complications In Spine Surgery. Sci Rep 12: 14350.
- Muñoz, M., Acheson, AG., Auerbach M et al. (2017) International Consensus Statement on The Peri-Operative Management of Anaemia and Iron Deficiency. Anaesthesia. 72: 233-247. [Crossref].
- Warner, SJ., Garner, MR., Nguyen, JT., Lorich DG (2016) Perioperative Vitamin D Levels Correlate With Clinical Outcomes After Ankle Fracture Fixation. Arch Orthop Trauma Surg. 136: 339-44. [Crossref].
- Skrobot, W., Liedtke, E., Krasowska K et al. (2019) Early Rehabilitation Program and Vitamin D Supplementation Improves Sensitivity of Balance and the Postural Control in Patients after Posterior Lumbar Interbody Fusion: A Randomized Trial. Nutrients 11. [Crossref].
- Ko, S., Chae, S., Choi, W., Kwon, J., Choi JY (2020) The Effectiveness of Vitamin D Supplementation in Functional Outcome and Quality of Life (QoL) of Lumbar Spinal Stenosis (LSS) Requiring Surgery. J Orthop Surg Res 15: 117. [Crossref].
- Metzger, MF., Kanim, LE., Zhao, L., Robinson, ST., Delamarter RB (2015) The Relationship Between Serum Vitamin D Levels And Spinal Fusion Success: A Quantitative Analysis. Spine (Phila Pa 1976) 40: 458-468. [Crossref].
- Bonjour JP (2011) Protein Intake And Bone Health. Int J Vitam Nutr Res. 81: 134-142. [Crossref].
- Khalooeifard, R., Oraee-Yazdani, S., Keikhaee, M., Shariatpanahi ZV (2022) Protein Supplement and Enhanced Recovery After Posterior Spine Fusion Surgery: A Randomized, Double-blind, Placebo-controlled Trial. Clin Spine Surg 35: E356-E362. [Crossref].
- Khalooeifard, R., Shariatpanahi, ZV., Ahani A et al. (2021) Effect of Protein Supplement on Paraspinal Muscles in Spine Fusion Surgery: A Randomized, Double-Blind, Placebo-Controlled Trial. Int J Spine Surg 15: 47-54. [Crossref].
- Monticone, M., Ferrante, S., Teli M et al. (2014) Management of Catastrophising and Kinesiophobia Improves Rehabilitation After Fusion for Lumbar Spondylolisthesis and stenosis. A Randomised Controlled Trial. Eur Spine J 23: 87-95. [Crossref].
- Madera, M., Brady, J., Deily S et al. (2017) The Role of Physical Therapy And Rehabilitation After Lumbar Fusion Surgery for Degenerative Disease: A Systematic Review. J Neurosurg Spine 26: 694-704. [Crossref].
- Brennan, GP., Shultz, BB., Hood, RS., Zahniser, JC., Johnson, SC., et al. (1994) The Effects of Aerobic Exercise After Lumbar Microdiscectomy. Spine (Phila Pa 1976) 19: 735-739. [Crossref].
- Abbott, AD., Tyni-Lenné, R., Hedlund R (2010) Early Rehabilitation Targeting Cognition, Behavior, And Motor Function After Lumbar Fusion: A Randomized Controlled Trial. Spine (Phila Pa 1976) 35: 848-857. [Crossref].
- Andersen, T., Christensen, FB., Egund N et al. (2009) The Effect of Electrical Stimulation on Lumbar Spinal Fusion in Older Patients: A Randomized, Controlled, Multi-Center Trial: Part 2: Fusion Rates. Spine (Phila Pa 1976) 34: 2248-2253. [Crossref].
- Kramer, M., Katzmaier, P., Eisele, R., Ebert, V., Kinzl L et al. (2001) Surface Electromyography-Verified Muscular Damage Associated With The Open Dorsal Approach To The Lumbar Spine. Eur Spine J 10: 414-420. [Crossref].
- Kramer, M., Völker, HU., Weikert E et al. (2004) Simultaneous Measurement of Intramuscular Pressure and Surface Electromyography of The Multifidus Muscle. Eur Spine J. 13: 530-536. [Crossref].
- Biering-Sørensen F (1984) Physical Measurements As Risk Indicators For Low-Back Trouble Over A One-Year Period. Spine (Phila Pa 1976) 9: 106-119. [Crossref].
- Tarnanen, S., Neva, MH., Kautiainen H et al. (2013) The Early Changes In Trunk Muscle Strength And Disability Following Lumbar Spine Fusion. Disabil Rehabil 35: 134-139. [Crossref].
- Cowan, JA., Dimick, JB., Wainess, R., Upchurch, GR., Chandler WF et al. (2006) Changes In The Utilization of Spinal Fusion in The United States. Neurosurgery 59: 15-20. [Crossref].
- Cutshall, SM., Wentworth, LJ., Engen, D., Sundt, TM., Kelly RF (2010) Effect of Massage Therapy on Pain, Anxiety, And Tension In Cardiac Surgical Patients: A Pilot Study. Complement Ther Clin Pract 16: 92-95. [Crossref].
- Keller G (2012) The Effects of Massage Therapy After Decompression And Fusion Surgery of The Lumbar Spine: A Case Study. Int J Ther Massage Bodywork. 2012;5(4):3-8. [Crossref].
- Scrimshaw, SV., Maher CG (2001) Randomized Controlled Trial Of Neural Mobilization After Spinal Surgery. Spine (Phila Pa 1976) 26: 2647-2652. [Crossref].
- Reyes, A., Aguilera, MP., Torres, P., Reyes-Ferrada, W., Peñailillo L (2021) Effects of Neural Mobilization in Patients After Lumbar Microdiscectomy Due To Intervertebral Disc Lesion. J Bodyw Mov Ther. Jan 2021;25:100-107. [Crossref].
- Nielsen, PR., Jørgensen, LD., Dahl, B., Pedersen, T., Tønnesen H (2010) Prehabilitation And Early Rehabilitation After Spinal Surgery: Randomized Clinical Trial. Clin Rehabil 24: 137-148. [Crossref].
- Özden F (2022) The Effectiveness of Physical Exercise After Lumbar Fusion Surgery: A Systematic Review and Meta-Analysis. World Neurosurg 163: 396-412. [Crossref].
- Quek, JMT., Tan, J., Toh I et al. (2022) Factors Associated with Pain Intensity and Walking Disability After Lumbar Fusion: A Longitudinal Study. Spine (Phila Pa 1976) 47: 597-606. [Crossref].
- Bogaert, L., Thys, T., Depreitere B et al. (2022) Rehabilitation to improve outcomes of lumbar fusion surgery: a systematic review with meta-analysis. Eur Spine J. 31: 1525-1545. [Crossref].



