Background: Fungal sepsis is a major cause of mortality in intensive care units (ICUs), particularly in resource-limited countries where access to diagnostics and antifungal treatments is restricted. In the Democratic Republic of the Congo (DRC), data on these infections remain scarce. This study aims to identify the fungal pathogens responsible for sepsis in ICUs and evaluate their antifungal susceptibility profiles.
Methods: This was a prospective multicenter study conducted between 2022 and 2023 in three hospitals in Lubumbashi. ICU patients suspected of fungal sepsis were included. Biological samples were analyzed for fungal identification and antifungal susceptibility testing. Clinical and demographic data, including comorbidities and infection entry sites, were collected and analyzed using SPSS and Excel software.
Results: Among 33 patients, the mean age was 49.14 ± 22.29 years, with a male predominance (57.6%). The most common comorbidities were hypertension (24%) and diabetes (15.5%). The primary infection entry sites were the genitourinary tract (67%), lungs (60%), and multiple-site infections (58%). The most frequently isolated fungal species were Candida albicans (50.17%), followed by non-albicans Candida species (45.3%) and Aspergillus spp. (4.51%). Antifungal susceptibility testing revealed a high resistance of non-albicans Candida to fluconazole (77.8%), whereas C. albicans remained susceptible to amphotericin B (86.4%), nystatin (77.3%), and caspofungin (63.6%). The overall mortality rate was 71.43%, with 75% of deaths occurring within the first five days and more than 80% after ten days of hospitalization.
Conclusion: Fungal infections in ICUs in Lubumbashi are predominantly caused by Candida albicans, with significant antifungal resistance observed in non-albicans Candida species and a high mortality rate. Optimized management, including early diagnosis, rigorous microbiological surveillance, and improved antifungal stewardship, is crucial to improving patient outcomes in intensive care.
Fungal Sepsis; Candida; Antifungals; Resistance; Intensive Care; Lubumbashi
Sepsis is a severe and often fatal syndrome caused by the microbial invasion of normally sterile body sites. It is characterized by a dysregulated immune response to infection, leading to organ failure and systemic disturbances, including immune, endocrine, cardiovascular, and metabolic dysfunctions, which significantly impact patient prognosis[1, 2]. While bacterial infections remain the primary cause of sepsis in intensive care units (ICUs), the incidence of fungal infections, particularly candidemia, has increased in recent years, posing a major challenge in ICUs, especially in Lubumbashi, Democratic Republic of the Congo (DRC). The diagnosis and management of these infections are complicated by the diversity of fungal pathogens and the limited availability of effective antifungal treatments [3].
Critically ill patients in intensive care units (ICUs), often immunocompromised, are particularly vulnerable to fungal infections, some of which can be severe. The causative agents include genera such as Candida, Aspergillus, Cryptococcus, and other opportunistic molds [4–7]. These infections pose a significant global health threat, particularly among critically ill ICU patients, even in the absence of traditional risk factors. They contribute substantially to morbidity, mortality, prolonged hospital stays, and increased healthcare costs [8, 9] In Lubumbashi, the diagnosis and treatment of these infections are hindered by infrastructure challenges, the lack of appropriate diagnostic tests, limited availability of antifungal drugs, and inadequate antifungal stewardship.
Fungal sepsis is a growing concern in intensive care units (ICUs) worldwide, particularly in resource-limited countries. According to recent studies, Candida albicans and Aspergillus fumigatus are the most frequently identified fungal pathogens in critically ill patients. However, other less common fungi, such as Mucor and Cryptococcus neoformans, are also responsible for severe sepsis, particularly in immunocompromised patients, including those with HIV, diabetes, or undergoing immunosuppressive therapy [10].
Early identification of fungal infections remains a major challenge in developing countries due to the limited availability of specialized diagnostic tests (fungal culture, PCR, antigen testing) [11, 12]. Additionally, the management of fungal sepsis relies on the administration of appropriate antifungal agents; however, in low-resource settings, access to broad-spectrum antifungals such as amphotericin B or azoles is often restricted. The increasing antifungal resistance observed, particularly in Candida albicans, Aspergillus, and Cryptococcus, further complicates treatment strategies [13].
The disruption of the mucosal or skin barrier induced by sepsis, along with impaired neutrophil production and function, significantly increases vulnerability to fungal infections. Cellular immunity dysfunction, metabolic disturbances, and aging further weaken the body's defense mechanisms, increasing the risk of fungal infections[14]. Additional factors compromising immunity include prolonged surgical interventions, long-term use of broad-spectrum antibiotics, cytotoxic chemotherapy, immunosuppressive therapy in transplantation, intravenous nutrition, multiple-lumen catheters, renal replacement therapy, and mechanical ventilation [15].
From an epidemiological perspective, fungal infections exhibit geographic and temporal variability [16]. A large-scale study on the prevalence of infections in European ICUs reported that fungal infections accounted for 16% of all infections, with Candida spp. and Aspergillus spp. being the most frequently isolated pathogens [16]. Similarly, fungal sepsis is prevalent in sub-Saharan Africa [17], though the true burden of severe fungal infections remains largely unknown. According to various studies, the primary risk factors for fungal diseases in Africa include HIV/AIDS, tuberculosis, poverty, and an increasing number of patients with non-communicable diseases such as diabetes [17, 18].
In an Egyptian ICU study, fungal infections accounted for 1% of all infections [19]. As in many resource-limited African countries, data on fungal infections in the Democratic Republic of the Congo (DRC) are scarce. One review estimated a 5.4% prevalence of severe fungal infections, with 6,168 annual cases of cryptococcal meningitis, 2,800 cases of Pneumocystis jirovecii pneumonia, and 380 cases of invasive aspergillosis in HIV patients. Oral and esophageal candidiasis affect 50,470 and 28,800 HIV-positive patients, respectively, while recurrent vulvovaginal candidiasis and tinea capitis are estimated at 1,202,640 and 3,551,900 cases, respectively [20]. However, fungal sepsis has received less attention, despite accounting for approximately 20% of sepsis cases with a mortality rate ranging from 40% to 70% [21,22]. In Lubumbashi, factors such as a hot and humid climate, a high prevalence of immunocompromised individuals (diabetes, HIV, malnutrition), and unrestricted access to antibiotics contribute to the high burden of fungal infections [23]. Early diagnosis is challenging, as symptoms are often nonspecific, overlapping with bacterial infections, and co-infections further complicate diagnostic accuracy [14].
Despite ICUs representing only 5% of hospital beds, they account for over 20% of nosocomial infections [14]. Fungal infections, which are both frequent and often fatal, commonly affect ICU patients. The Surviving Sepsis Campaign recommends broad-spectrum empirical antifungal therapy to reduce mortality [24], yet no precise criteria exist for initiating antifungal treatment. Early antifungal use remains limited due to concerns about cost, side effects, and resistance. When antibiotic therapy fails, fluconazole is commonly introduced, although antifungal resistance is an increasing concern [25, 26].
In Lubumbashi, despite fungal sepsis being a leading cause of mortality, few studies have investigated its etiology and antifungal susceptibility patterns. This study aims to address the knowledge gap on fungal sepsis in Lubumbashi, where healthcare infrastructure remains limited. Identifying causative pathogens and analyzing their antifungal susceptibility is crucial to optimizing treatment strategies and reducing mortality. These findings could inform local policies and resource allocation for fungal sepsis management. The primary objective of this study is to determine the etiological agents of fungal sepsis in ICUs, evaluate their antifungal susceptibility, and analyze clinical outcomes.
Study Design, Setting, and Period
This was a cross-sectional, prospective, multicenter, and observational study conducted in the intensive care units (ICUs) of three hospitals in Lubumbashi, Democratic Republic of the Congo (DRC). The primary objective was to analyze the clinical and etiological characteristics as well as the antifungal susceptibility profiles of fungal microorganisms responsible for sepsis in ICUs.
Study Hospitals
The study was conducted in three hospitals in Lubumbashi: Clinique Universitaires de Lubumbashi (CUL), Hôpital de la SNCC, and Clinique Diamant de Lubumbashi (CMDL). These hospitals were selected based on the presence of at least one anesthesiologist-intensivist, a high patient admission rate, a strong referral capacity, and the availability of basic intensive care equipment.
- Cliniques Universitaires de Lubumbashi (CUL) is a tertiary-level health facility with 211 functional beds out of a total capacity of 230 beds. The ICU, part of the Anesthesia and Intensive Care Medicine Department, has six beds (3% of the hospital’s total capacity) and is staffed by four anesthesiologist-intensivists, four interns, eight nurses, and one auxiliary staff member.
- Hôpital de la Société Nationale des Chemins de Fer du Congo (SNCC) is a semi-public facility primarily serving railway employees but also functioning as a general referral hospital. It has 200 functional beds out of a total of 250 beds, with an ICU comprising eight beds (4% of the hospital’s capacity). The unit operates with one anesthesiologist-intensivist, five nurses, and one auxiliary staff member.
- Clinique Diamant de Lubumbashi (CMDL) is a private hospital with 65 beds, including four beds in the continuous care unit and eight beds in the ICU, representing 12% of the hospital's total capacity. The ICU is managed by eight physicians, including one anesthesiologist-intensivist, and eight nurses.
These ICUs receive patients from various specialties, including emergency medicine, surgical and medical units, and postoperative cases from elective and emergency surgeries (visceral surgery, trauma, obstetrics-gynecology, neurosurgery, and urology). They also manage critically ill patients requiring intensive care due to surgical, medical, or traumatic conditions. Each ICU is staffed with at least one intensivist specialist, and on-call duties are provided by a resident physician under the supervision of a senior intensivist.
Study Population
The study included patients with clinical signs of sepsis, defined by an infection confirmed by fungal culture and meeting the Sepsis-3 diagnostic criteria[27]. The study was conducted in the ICUs and/or high-dependency units of the selected hospitals during the study period. Patients diagnosed with sepsis and requiring intensive treatment and monitoring received care based on the 2021 Surviving Sepsis Campaign (SSC) guidelines.
Inclusion Criteria
Patients meeting the following criteria were included:
- Suspected sepsis upon admission or during the ICU stay.
- Age ≥ 16 years, with suspected sepsis or septic shock.
- Hospitalized in the ICU or continuous care unit of the selected hospitals.
Exclusion Criteria
The study excluded patients with the following characteristics:
- Age < 16 years.
- Death within 24 hours of hospital admission.
- Pregnancy.
- Readmission for sepsis or septic shock during the study period.
- Lack of informed consent for study participation.
Sampling Technique
A non-exhaustive sampling approach was used. The sample size and study duration were determined based on staffing and financial constraints. Data on total admissions and the number of patients during the study period were collected from the hospital data management system. At the end of data collection, 33 cases of fungal sepsis were identified.
General Data Collection Process
Data were collected consecutively and recorded in a standardized data collection form. A quality control process was conducted by the research team before data encoding using Microsoft Excel 365®. Data were categorized into independent and dependent variables.
Demographic and Clinical Data
Demographic variables included age, sex, and comorbidities to evaluate their impact on fungal sepsis outcomes. Clinical data encompassed the reason for ICU admission, the source of ICU admission (emergency department, internal medicine, surgery, obstetrics-gynecology), time to ICU admission, type of admission (elective or emergency), vital signs, suspected infection source, and therapeutic interventions. These interventions included mechanical ventilation, catheterization, and blood transfusion, which are known risk factors for fungal infections in critically ill patients.
The quick Sequential Organ Failure Assessment (qSOFA) score was used to assess disease severity upon admission, as it helps predict the likelihood of poor outcomes in sepsis patients. The collected clinical and demographic data aimed to identify potential risk factors, patient characteristics, and ICU management strategies associated with fungal sepsis.
Culture
Samples were cultured on Sabouraud agar with chloramphenicol to inhibit bacterial growth and incubated at 35–37°C for 24 hours.
Identification
Fungal isolates were identified using:
- Gram staining (to visualize spores, pseudohyphae, or hyphae under ×100 magnification).
- Lactophenol blue staining (to observe fungal structures under ×40 magnification).
- Germ tube test (to differentiate Candida albicans based on germ tube formation without constriction at the base).
- Biochemical testing using the Integral System Yeasts Plus (Liofilchem® ref. 71822).
Antifungal Susceptibility Testing
After the identification of fungal agents responsible for sepsis, an antifungal susceptibility test was performed. The antifungigram was conducted using the agar diffusion method (Kirby-Bauer method) with discs containing various antifungal agents, including fluconazole, amphotericin B, voriconazole, itraconazole, flucytosine, caspofungin, ketoconazole, miconazole, and nystatin. The interpretation was carried out following the manufacturer’s recommendations provided with the discs (Liofilchem®).
Statistical Analysis
A database containing demographic, clinical, and mycological characteristics was created and analyzed using IBM SPSS Statistics v. 29.0 (IBM Corp., Armonk, NY, USA). Exploratory and descriptive data analyses were conducted to identify patterns across variables. Categorical variables were expressed as frequencies and percentages.
Ethical Considerations
The study protocol was approved by the Ethics Committee of the University of Lubumbashi under reference number UNILU/CEM/030/2021. Approval letters were also obtained from hospital directors. Voluntary informed consent was obtained from each patient before participation, following the Helsinki Declaration guidelines. Confidentiality and anonymity were strictly maintained during data collection and processing by the research team.
Characteristics of the study population
Table 1 presents the general characteristics of the 33 patients included in the study. The age distribution of the patients ranges from 16 to over 60 years old, with a balanced distribution: 36.4% are aged between 16 and 39 years, 27.2% between 40 and 60 years, and 36.4% are over 60 years old. The mean age is 49.14 ± 22.29 years. Regarding gender, most patients are male (57.6%), while 42.4% are female. In terms of origin, most patients were referred from health centers (CS transfers) (48.5%), followed by emergency admissions (27.3%). Other sources, such as surgery, internal medicine, and neurology, were less frequent, each accounting for 6.1% of cases. Concerning the time of admission, 69.7% of patients were admitted within 7 days, whereas 33.3% were admitted after more than 7 days. Finally, based on medical specialization, 72.7% of patients were medical cases, followed by surgical cases (18.2%) and gynecological-obstetric cases (9.1%).
Table 1: General Patient Characteristics.
|
N=33 |
% |
Age |
16-39 years old |
12 |
36.4 |
40-60 years old |
9 |
27.2 |
˃60 years |
12 |
36.4 |
Middle Ages |
49.14±22.29 years |
Sex |
|
|
F |
14 |
42.4 |
M |
19 |
57.6 |
Origin |
Surgery |
2 |
6.1 |
Internal Medicine |
2 |
6.1 |
Neurology |
2 |
6.1 |
CS Transfer |
16 |
48.5 |
Emergency room |
9 |
27.3 |
Admission time |
˃7 |
10 |
33.3 |
˃7 |
23 |
69.7 |
Patient Type |
|
|
Surgical |
6 |
18.2 |
Gyneco-Obstétrical |
3 |
9.1 |
Medical |
24 |
72.7 |
Clinical and paraclinical characteristics of the patients studied
Table 2 presents the clinical and paraclinical characteristics of patients with sepsis. Among the comorbidities, hypertension (24%) and diabetes (15.5%) are the most prevalent conditions. Genitourinary tract infections (67%) and pulmonary infections (60%) are the most common sites of infection. Regarding vital signs, patients exhibit moderate hypoxia (oxygen saturation at 90.03%) and tachycardia (heart rate of 109.12 bpm). The average Glasgow Coma Scale (GCS) score of 11.24 indicates impaired consciousness in many patients. Laboratory findings reveal moderate anemia, elevated white blood cell count (15,013.64/mm³), high lactate levels (3.80 mEq/L), and elevated creatinine (4.03 mg/dL), suggesting renal impairment and a significant inflammatory response.
Table 2: Clinical features, and paracliniq laboratory values of patients with sepsis.
Comorbidity (%) |
|
Alcohol |
6 |
COPD |
6 |
Cancer |
6 |
C.puerperal |
9 |
Diabetes |
15.5 |
HTA |
24 |
Obesity |
3 |
Sinusitis |
3 |
Site d’infection (%) |
|
Digestive |
3 |
Pulmonary |
60 |
Genitourinary |
67 |
Undetermined |
3 |
Multisite |
58 |
Vital Signs at Presentation |
|
Score de Glasgow (%) |
11.24±2.9 |
Oxygen saturation (%) |
90.03±10.2 |
Temperature (°C) |
36.788±1.5 |
Systolic blood pressure (mmHg) |
116.56±392 |
Diastolic blood pressure (mmHg) |
69,62±22,2 |
Mean arterial pressure (mmHg) |
82.7±24.5 |
Heart rate (beats/min) |
109.12±20.5 |
Respiratory rate (breathing/min) |
29.39±8.2 |
Laboratory baseline values |
|
Hémoglobine(g/dl) |
10.69 ± 2.45 |
Haematocrit |
32.27 ± 8.43 |
Globules blancs ( /mm3) |
15013.64±6273.5 |
Inserts ( /mm3) |
1152575.76±116595.5 |
Serum creatinine (mg/dl) |
4.03±3.9 |
Natremia(mEq/l) |
139.37±7.2 |
Kaliemia (mEq/l) |
4±0.867 |
Lactate (mEq/l) |
3.80 ± 3.531 |
Blood urea (mg/dl) |
37.03 ± 26.29 |
Bilirubine totale (mg/dl) |
3.08 ± 5.2 |
PaCO2 (mmHg) |
38.31 ± 12.6 |
Arterial Blood pH |
7.37 ± 0.16 |
PaO2 / FiO2 |
232.9 ± 122.64 |
Glycémie (mg/dl) |
183.32±146.4 |
Microorganisms isolated and identified after culture
A sample of blood, sputum, or body fluids was collected for microbiological culture from 79 patients suspected of developing sepsis. Among the results, 39% of patients were diagnosed with fungal sepsis (Figure 1). The distribution of fungal microorganisms was as follows: Candida albicans (50.17%), Non-albicans Candida (45.3%) including, Candida guilliermondii (6.77%), Candida famata (20.48%), fraunde (4.51%), Candida glabrata (6.77%), Candida krusei (6.77%) and Aspergillus spp. (4.51%) [Figure 2].
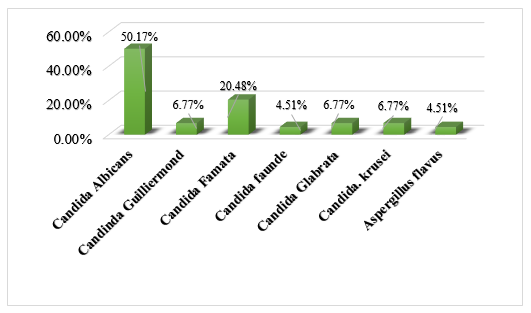
Figure 1: Distribution of fungal species
The antifungal susceptibility profiles
The fungi identified in this study were tested for specific antifungal agents, and their susceptibility to different antifungals was assessed. Figure 3 presents the results, revealing significant variability in susceptibility and resistance rates. Amphotericin B and Nystatin were found to be the most effective antifungals, both showing susceptibility rates of 75.76%, particularly against C. albicans (88.24% for Amphotericin B and 70.59% for Nystatin) and C. famata (60% and 80%, respectively). Conversely, high resistance was observed for several azole-class antifungals, including Ketoconazole (81.82% resistance), Fluconazole (69.70%), and Clotrimoxazole (66.67%). Among the fungal species, C. glabrata exhibited particularly high resistance, with an average resistance rate of 71.43%, while C. krusei showed variable sensitivity depending on the antifungal tested. C. guilliermondii and C. faunde displayed intermediate susceptibility profiles, with average sensitivity rates of 47.37% and 55%, respectively.
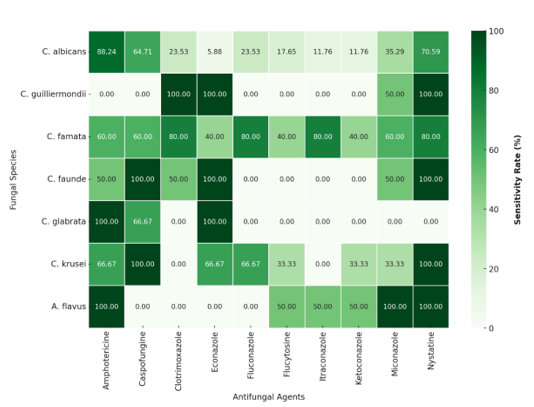
Figure 2: Susceptibility profile of fungal species to the antifungals tested
Outcomes
The analysis of clinical outcomes in ICU patients diagnosed with fungal sepsis highlights a concerning mortality rate. As shown in Figure 1, 71.43% of patients succumbed to the infection, while only 28.57% survived. Among the deceased patients, the majority (approximately 75%) died within the first 5 days of hospitalization. However, between 5 and 10 days, the mortality rate declines, with a more balanced distribution between survival and death. In contrast, for patients with prolonged hospitalization exceeding 10 days, mortality remains alarmingly high, accounting for over 80% of cases.
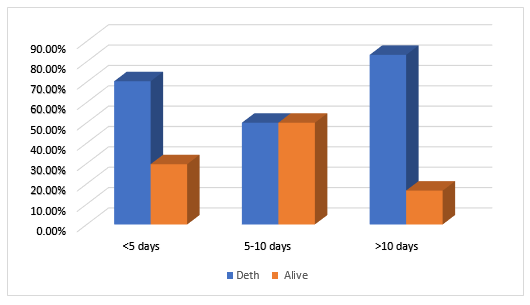
Figure 3: Outcomes as a function of time
Epidemiological characteristics of patients
An overview of the general characteristics of ICU patients with fungal sepsis included 33 cases, showing a diverse age distribution. A notable proportion of younger patients (36.4%) fell within the 16–39 age group, while an equivalent percentage (36.4%) were over 60 years old. These findings align with studies conducted in Indonesia, where the age range was between 18 and 79 years, with a mean age of 58.0 years [28], and in India, where the age range was between 18 and 80 years, with an average age of 43.5 years [29]. Similarly, another study reported that over a quarter of fungal sepsis cases involved patients aged over 60 years [14]. Theoretically, advanced age is a recognized risk factor for fungal infections, primarily due to the progressive decline in host immune defense mechanisms, which may be exacerbated by pathogen virulence, immune system weakening, or prolonged ICU stays [14, 30].
In the current study, 42.5% of patients were female, while 57.6% were male. These findings are consistent with existing literature, which suggests that men have a 1.4 to 3.5 times higher risk of developing invasive fungal infections, including sepsis, compared to women [14, 30, 31]. However, the relationship between gender and susceptibility to fungal infections remains complex, extending beyond the scope of our analysis. While male predominance appears to be a trend, the difference is not strong enough to be considered a sole determining factor. One hypothesis suggests that men may have a higher predisposition to urinary tract infections (UTIs), which could contribute to the development of fungal sepsis[14]. Additionally, genetic factors linked to sex, differences in environmental exposure, and more frequent risk behaviors among men—such as less balanced diets, less rigorous health habits, or increased use of immunosuppressive substances—may further explain this heightened vulnerability [32,33]. This suggests that men may be more prone to invasive fungal infections due to a combination of biological and socio-environmental factors. As a result, the relationship between sex and susceptibility to fungal infections requires further investigation to better understand the underlying mechanisms.
In the current study, most patients (45%) were admitted through transfers from other medical institutions, followed by 27.3% from the emergency department. This suggests that a significant proportion of patients required specialized, urgent, or complex care, highlighting the severity of their clinical conditions. The high rate of admissions from transfers and emergency services indicates that many of the cases studied were related to critical medical emergencies, necessitating intensive monitoring and specialized interventions.
Regarding time to admission, most patients were admitted within 7 days, which may indicate effective early case management, a crucial factor for improving prognosis, particularly in urgent conditions. However, one-third of patients were admitted after more than 7 days, which may reflect delayed management, a more complex clinical course, or diagnostic challenges. Studies have demonstrated that delayed admission of sepsis patients is associated with increased mortality and prolonged hospital stays [34,35]. Consequently, the Surviving Sepsis Campaign (SSC) guidelines recommend that adults with sepsis requiring ICU admission should be admitted within 6 hours to optimize outcomes [24]. The progression of sepsis, from initial symptoms to ICU admission, varies among patients. However, in low-resource settings such as the Democratic Republic of the Congo (DRC), several factors—limited access to primary healthcare, poverty, lack of awareness, and cultural misconceptions—contribute to delayed ICU admission, potentially worsening patient outcomes.
Finally, the predominance of medical patients (72.7%) compared to surgical (18.2%) and gynecological-obstetric (9.1%) patients highlights a primarily medical clinical profile, suggesting that the study focuses on internal pathological conditions, often chronic or complex. This distribution may reflect the nature of care provided in the studied institutions, where medical cases are more prevalent. Additionally, it may influence therapeutic and management strategies, particularly for patients with complex medical diseases, requiring specialized and long-term intensive care interventions.
Clinical characteristics of the population
Clinically, the present study revealed that the most common comorbidity among patients with fungal sepsis was arterial hypertension (24%), followed by diabetes (15.5%) and conditions related to the puerperal context (9%). These findings are consistent with previous studies. For instance, in India, diabetes was reported as the most common comorbidity (30%) associated with fungal infections leading to sepsis [14]. Similarly, in the study by Tirath Singh et al., diabetes was the most frequently observed comorbidity, affecting 28.6% of patients with fungal infections [29]. The presence of multiple comorbidities, particularly age-related conditions, significantly increases the risk of invasive fungal infections. This increased risk can be attributed to the alteration of immune mechanisms and the presence of predisposing factors such as hypertension and diabetes, which are well-known for their detrimental effects on overall health and their association with various complications [33]. This distribution of comorbidities highlights a patient profile with significant cardiovascular and metabolic risk factors. Hypertension and diabetes are well-established risk factors for the development of serious infections, including fungal infections. Additionally, puerperal conditions may also contribute to the vulnerability of patients to fungal infections, further complicating clinical management. Overall, these findings emphasize the importance of considering underlying comorbidities—including cardiovascular and metabolic diseases, as well as puerperal conditions—in the clinical management of fungal sepsis patients. Tailored and individualized care strategies are essential to improving clinical outcomes in these high-risk populations.
The results of this study highlight the most frequently observed sites of infection, with genitourinary tract infections (67%) and pulmonary infections (60%) being the most common. These findings are consistent with existing data on nosocomial and community-acquired infections, particularly in patients with comorbidities such as diabetes and hypertension. Critically ill patients, especially those with urinary catheters, are at an increased risk of developing fungal infections [6,36]. Additionally, fungal lung infections, which are often underdiagnosed, may contribute to the high frequency of respiratory infections observed in this study [37,38]. ICU patients, who often have compromised immune systems, are particularly vulnerable to these infections [37]. Another notable finding of this study is the high prevalence of multiple infections (58%), indicating that many patients present with complex pathologies requiring specialized therapeutic approaches and interdisciplinary coordination. These findings emphasize not only the diversity of infection sites but also the complexity of their management, necessitating integrated therapeutic strategies to improve the prognosis of critically ill patients.
Vital signs indicate moderate hypoxia, with an oxygen saturation of 90.03%, and an elevated heart rate of 109.12 beats per minute, which may reflect a physiological response to hypoxia or another form of systemic stress. Hypoxia is a common pathophysiological alteration in sepsis, often driven by accentuated inflammatory responses that increase vascular permeability, leading to acute pulmonary edema and the subsequent development of acute respiratory distress syndrome (ARDS) [39,40].
The mean Glasgow Coma Scale (GCS) score of 11.24 indicates impaired consciousness, suggesting possible neurological deterioration or confusion, which is frequently observed in critically ill patients with severe infections. This finding aligns with other studies, such as one conducted in Cameroon, which reported an average GCS score of 9 ± 4.3 [41]. However, it contrasts with a study in Jordan, where the majority of patients had an average GCS score between 13 and 14 [42].
Laboratory results indicate moderate anemia, elevated white blood cell count (15,013.64/mm³), elevated lactate levels (3.80 mEq/L), and elevated creatinine levels (4.03 mg/dL), suggesting renal failure and a significant inflammatory response. These biological abnormalities are key indicators of sepsis severity, illustrating the systemic response of the body to infection. The anemia observed in these patients is typically inflammatory anemia, which is common in acute infection-related responses. This form of anemia is primarily driven by pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6), which inhibit the production of erythropoietin, a hormone essential for red blood cell production. Additionally, these cytokines increase hepcidin levels, a protein that regulates iron absorption and distribution. The elevated hepcidin levels restrict iron availability, further impairing red blood cell production and contributing to the anemia commonly seen in septic patients [43, 44].
Elevated lactate is a critical clinical marker in critically ill patients, particularly those with sepsis or septic shock. It is widely used as a prognostic indicator of infection severity, as increased lactate levels are strongly associated with higher mortality rates [45,46]. This elevation in lactate often reflects tissue hypoxia, which results from impaired organ perfusion due to systemic hypotension and vascular dysfunction in sepsis[40]. As a result, lactate serves as a valuable biomarker for assessing sepsis severity and monitoring the patient’s response to treatment.
Finally, acute kidney injury (AKI) is a common and potentially fatal complication of sepsis, typically resulting from renal hypoperfusion caused by hypotension and circulatory shock. AKI significantly worsens patient outcomes, increasing mortality by six to eight times. The kidneys are among the first organs affected in sepsis, with approximately two-thirds of patients with septic shock developing AKI. In many cases, renal dysfunction occurs even before emergency admission, underscoring the critical importance of early detection and prompt management of kidney function in septic patients [47, 48].
These observations emphasize the critical importance of early and targeted management of sepsis patients, particularly in intensive care units (ICUs). Rigorous monitoring of key biological parameters, such as lactate and creatinine levels, is essential for assessing disease severity and guiding treatment decisions. Additionally, the proactive management of complications, including anemia and acute kidney injury (AKI), plays a crucial role in improving patient outcomes and reducing sepsis-related mortality.
Microorganisms identified in culture
Infections that can progress to sepsis are caused by a wide variety of microorganisms, with their distribution varying by healthcare setting and geographical location[49]. While bacterial infections are widely recognized as the most common causes of sepsis in intensive care units (ICUs), the incidence of invasive fungal infections has been steadily increasing [3, 50]. In this study, 39% of sepsis patients had a fungal infection, a rate significantly higher than the global average reported in the literature, where fungal infections account for approximately 15% of sepsis cases[51]. A 24-hour prevalence survey conducted in 1, 150 centers across 88 countries found that fungal infections were responsible for 16% of ICU infections [16, 48], a finding consistent with a study in India, which reported a 15% incidence of fungal sepsis [52]. The higher rate of fungal infections observed in this study does not yet have a definitive explanation. However, the prevalence of fungal infections varies depending on geographical factors and healthcare resources. It is possible that local environmental conditions in Lubumbashi, as well as the severity of patient cases, contributed to this elevated incidence. These findings highlight the urgent need for enhanced surveillance and targeted management strategies for fungal infections in ICUs, particularly in resource-limited settings where their burden may be underestimated or inadequately addressed.
In this study, Candida spp. was the most frequently identified fungal pathogen (88.7%), followed by Aspergillus spp. (4.51%) among ICU patients with fungal infections. These findings align with existing literature, which recognizes Candida as the leading cause of fungal infections in critically ill patients, whereas Aspergillus infections account for 0.3% to 19% of cases. Despite this relatively low incidence, Aspergillus infections are often associated with high morbidity and mortality rates, even in the absence of conventional risk factors [53, 54].
However, these results contrast with the findings of Durga et al., who reported a higher prevalence of aspergillosis. This discrepancy was attributed to the use of broader diagnostic criteria for aspergillosis and the presence of specific risk factors, such as chronic lung disease, liver disease, autoimmune disorders, and increased exposure to Aspergillus in hospital environments under construction [3, 55].
The rapid and accurate identification of Candida spp. is crucial for the selection of appropriate antifungal agents and the optimization of patient management [56]. In this study, Candida albicans was the most frequently isolated species (50.17%) among fungal infections. The predominance of C. albicans over non-albicans Candida species varies depending on geographical regions and studied populations [57]. This dominance of C. albicans in critically ill patients has also been reported in several studies conducted in Algeria, Ethiopia, Colombia, and China [56, 58-61].
There is significant variation in the species distribution of C. albicans and non-albicans Candida species responsible for invasive candidiasis in intensive care units, depending on the healthcare facility and geographical region[14]. Although C. albicans remains the leading cause of fungal infections, an epidemiological shift favoring non-albicans Candida species has been observed in many global studies, with regional differences [62–64]. For instance, Ahmad et al. reported that non-albicans Candida species were dominant (72%), surpassing C. albicans [14]. Similarly, Hasan M. Al-Dorzi et al. in Saudi Arabia found that fungal infections accounted for 56.2% of ICU cases, while Lourdes Rodriguez in Peru reported that 71.9% of cases were caused by non-albicans Candida species albicans [30, 65].
Among the non-albicans Candida species, Candida famata was the most frequently isolated species (20.48%) in this study. In contrast, studies conducted in South America reported Candida parapsilosis as the most common species, followed by Candida tropicalis, with a gradual increase in Candida glabrata and the emergence of Candida auris [64]. Similarly, C. tropicalis and C. krusei were the most frequently identified species in studies conducted in Saudi Arabia [64], Iran [66], and Ethiopia [56]. The global distribution of Candida species remains poorly understood, as several factors may influence species prevalence. These include climate conditions, antifungal usage in healthcare settings, and the regional microbiota of patients, all of which play a role in shaping the epidemiological profile of invasive candidiasis [14, 63].
The antifungal susceptibility profiles
In the present study, the results indicate that amphotericin B and nystatin were the most effective antifungals, with a sensitivity rate of up to 75.76%. These findings confirm previous research, which highlights their continued effectiveness despite the emergence of resistant strains [67]. Regarding C. albicans (88.24% sensitivity to amphotericin B) and C. famata (80% sensitivity to nystatin), these results are particularly noteworthy, reinforcing their relevance as first-line treatment options. In contrast, high resistance rates were observed for azole antifungals, particularly ketoconazole (81.82%), fluconazole (69.70%), and clotrimoxazole (66.67%), findings that are consistent with previous studies. The increasing resistance to azoles has been widely reported, especially for C. glabrata and C. krusei, which are known for their intrinsic or acquired resistance to fluconazole and other azoles krusei[68,69]. Additionally, the variability in the sensitivity of C. krusei and the intermediate resistance profiles of C. guilliermondii and C. faunde (47.37% and 55% mean sensitivity, respectively) underscore the importance of continuous antifungal susceptibility monitoring. These findings highlight the need for treatment adaptation based on local resistance profiles, reinforcing the importance of routine antifungal resistance surveillance and tailored therapeutic strategies [14,26].
Outcomes
Our results reveal a high mortality rate (71.43%) among ICU patients with fungal sepsis, with 75% of deaths occurring within the first five days and over 80% after ten days. This high fatality rate is largely attributable to late diagnosis, delayed management, and the emergence of antifungal-resistant strains [8,14,70]. Comorbidities such as hypertension (24%) and diabetes (15.5%) weaken patients by compromising immune responses and worsening the course of sepsis[8]. Additionally, genitourinary tract infections (67%) and pulmonary infections (60%), which are the primary infection entry points, often contribute to delays in initiating effective antifungal treatment. While Candida albicans remains the dominant species (50.17%), non-albicans Candida species (45.3%) exhibit high resistance to fluconazole (77.8%), limiting treatment options and worsening patient prognosis [71]. Acute kidney injury (elevated creatinine at 4.03 mg/dL) is an additional aggravating factor, contributing to multi-organ failure and reduced antifungal clearance [47]. Furthermore, persistent hypotension and elevated lactate levels (3.80 mEq/L) are indicators of septic shock, which is strongly associated with poor patient outcomes [45].
Although mortality decreases between days 5 and 10 of hospitalization, likely reflecting better responses to early treatments, it rises above 80% beyond 10 days, mainly due to nosocomial complications, secondary resistant infections, and antifungal treatment failure[68,69]. Delayed management and initial treatment failure are key contributing factors, further exacerbated by antifungal resistance and limited access to echinocandins [45]. This situation underscores the urgent need for early and optimized management, including rigorous microbiological monitoring, adaptation of antifungal therapy to local resistance patterns, and close patient monitoring to improve prognosis and reduce fungal sepsis-related mortality in ICUs [8, 9].
Fungal sepsis, particularly in intensive care settings, remains a complex and unpredictable pathology, often associated with high morbidity and mortality. This study found a high prevalence of fungal sepsis (39%), exceeding reported rates in the literature, with Candida spp. identified as the primary etiological agent, predominantly Candida albicans and Candida famata. The patient characteristics revealed a wide age distribution, a higher infection rate in men, and a high prevalence of comorbidities, particularly hypertension and diabetes, both of which are known to increase the risk of sepsis. Most patients were admitted within a short timeframe, suggesting effective management, although delays in admission were observed in some cases. The most frequent sites of infection were genitourinary (67%) and pulmonary (60%), which are often challenging to treat, particularly due to the high rate of multiple infections in critically ill patients. The antifungal susceptibility profile demonstrated notable efficacy of nystatin, amphotericin B, and caspofungin against Candida albicans, while a trend toward fluconazole resistance was observed, particularly among non-albicans Candida species.
These findings highlight the critical importance of continuous surveillance and targeted management of fungal infections in ICUs, particularly in the context of rising antifungal resistance. Strengthening diagnostic capabilities, optimizing antifungal stewardship, and implementing rigorous monitoring protocols are essential strategies to improve patient outcomes and reduce fungal sepsis-related mortality.
Limitations of the study
This study has several limitations that limit the generalization of its results. First, the small sample size (33 patients) limits the statistical power and representativeness of the conclusions. Secondly, the lack of long-term follow-up of patients after their hospitalization does not allow us to evaluate the impact of treatments on survival and post-sepsis complications. In addition, the study relies solely on fungal culture methods, excluding more sensitive diagnostic tools such as PCR or antigen tests, which may underestimate some cases of fungal infections. In addition, the lack of multivariate analysis prevents the identification of independent mortality factors, and data on the treatments administered are insufficiently detailed to assess their effectiveness. Finally, as the study is limited to three hospitals in Lubumbashi, the results cannot necessarily be extrapolated to the whole country or to other regions with different epidemiological contexts.
Clinical implications
The findings of this study underscore the critical need for enhanced surveillance of fungal infections, particularly those caused by Candida albicans and non-albicans Candida species, in intensive care units (ICUs). The increasing resistance to antifungals, especially fluconazole, necessitates a re-evaluation of treatment protocols and the optimized use of more effective antifungals, such as amphotericin B and nystatin, to combat resistant strains.
An individualized treatment approach, guided by antifungal susceptibility testing, is essential to reduce mortality associated with fungal sepsis. Additionally, continuous clinician training and heightened awareness of the clinical signs of fungal sepsis, alongside improved diagnostic infrastructure for antifungal resistance testing, will significantly enhance infection management in ICUs. This is particularly crucial in resource-limited settings such as Lubumbashi, where early detection and targeted therapy can greatly improve patient outcomes and survival rates.
This study highlights the significant prevalence of fungal infections in sepsis cases in intensive care, with most patients infected with Candida spp., particularly Candida albicans. The findings emphasize the importance of early pathogen identification and prompt initiation of appropriate antifungal therapy to improve patient outcomes. The observed resistance to fluconazole and the diversity of fungal species involved in infections necessitate increased vigilance and targeted monitoring in intensive care units (ICUs). Given these challenges, further studies with larger sample sizes and comprehensive surveillance are required to better understand the epidemiology of fungal infections and optimize treatment strategies, particularly in resource-limited settings.
We sincerely thank the medical and laboratory staff of the participating hospitals, as well as the ICU teams for their support in patient management and data collection. Our gratitude also goes to the University of Lubumbashi and collaborating institutions for their assistance. Finally, we appreciate the patients and their families for their participation, which made this study possible.
MM Manika, HN-T Situakibanza, Rivain Fefe Iteke et LK Kapend have done the conceptualization, F M Mujing, IM Téta, YM Mwanza, NM Tshibwaya have collected the data. E I Kasamba, MN Kabamba, BN Barayiga, AK Tano and MM Manika, have written, reviewed and edited the manuscript. All authors read and approved the final manuscript.
The authors declare that this study received no specific funding from public, commercial, or non-profit organizations.
Data availability
The data used in this study are available upon request from the authors.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no conflicts of interest related to this study.
- Fang F., Zhang Y., Tang J (2019) Association of Corticosteroid Treatment with Outcomes in Adult Patients with Sepsis: A Systematic Review and Meta-analysis. JAMA Intern Med 179: 213-223
- Hing Chan JK., Leung YLE (2017) A Retrospective Study on the Compliance with Surviving Sepsis Campaign Guideline in Patients with Sepsis Admitted to Intensive Care Unit in Hong Kong. J Intensive Crit Care 3: 1-10.
- Durga CS., Gupta N., Soneja M (2018) Invasive fungal infections in critically ill patients: A prospective study from a tertiary care hospital in India. Drug Discov Ther 12: 363-367.
- Azim A., Ahmed A (2024) Diagnosis and management of invasive fungal diseases in non-neutropenic ICU patients, with focus on candidiasis and aspergillosis: a comprehensive review. Front Cell Infect Microbiol 14: 1-12.
- Ostrosky-Zeichner L., Al-Obaidi M (217) Invasive Fungal Infections in the Intensive Care Unit. Infect Dis Clin North Am 31: 475-487.
- Soriano A., Honore PM., Puerta-Alcalde P (2023) Invasive candidiasis: current clinical challenges and unmet needs in adult populations. J Antimicrob Chemother 78: 1569-1585.
- Sirga S., Bindu KH., MS R (2023) Invasive Fungal Infections in A Teritiary ICU in Hyderabad. Acta Sci Med Sci 7: 183-191.
- Bassetti M., Giacobbe DR., Agvald-Ohman C (2024) Invasive Fungal Diseases in Adult Patients in Intensive Care Unit (FUNDICU): 2024 consensus definitions from ESGCIP, EFISG, ESICM, ECMM, MSGERC, ISAC, and ISHAM. Intensive Care Med 50: 502-515.
- Xu H., Yu SY., Zhou ML (2019) Epidemiology and antifungal susceptibility patterns of invasive fungal infections from 2012 to 2014 in a teaching hospital in central China. Infect Drug Resist 12: 3641-3651.
- Garnacho-Montero J, Barrero-García I, León-Moya C (2024) Fungal infections in immunocompromised critically ill patients. J Intensive Med 4: 299-306.
- Dangarembizi R., Wasserman S., Hoving JC (223) Emerging and re-emerging fungal threats in Africa. Parasite Immunol 45: 1-13.
- Arastehfar A., Wickes BL., Ilkit M (2019) Identification of mycoses in developing countries. J Fungi 5: 1-23.
- Hassoun N., Kassem II/, Hamze M (2023) Antifungal Use and Resistance in a Lower-Middle-Income Country: The Case of Lebanon. Antibiotics 12: 1-14.
- Ahmad S., Kumar S., Rajpal K (2022) Candidemia Among ICU Patients: Species Characterisation, Resistance Pattern and Association with Candida Score: A Prospective Study. Cureus 14: 1-9.
- Poissy J., Damonti L., Bignon A (2020) Risk factors for candidemia: A prospective matched case-control study. Crit Care 24: 1-11.
- Vincent JL., Sakr Y., Singer M (2020) Prevalence and Outcomes of Infection among Patients in Intensive Care Units in 2017. JAMA - J Am Med Assoc 323: 1478-1487.
- Bongomin F., Fayemiwo SA (2021) Epidemiology of fungal diseases in Africa : A review of diagnostic drivers. Curr Med Mycol 7: 63-70.
- Rajasingham R., Smith RM., Park BJ (2017) Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 17: 873-881.
- Negm EM., Mowafy SMS., Mohammed AA (2021) Antibiograms of intensive care units at an Egyptian tertiary care hospital. Egypt J Bronchol 15: 1-15
- Kamwiziku GK., Makangara JCC., Orefuwa E (2021) Serious fungal diseases in Democratic Republic of Congo - Incidence and prevalence estimates. Mycoses 64: 1159-1169.
- Boussina A., Ramesh K., Arora H (2023) Differentiation of Fungal, Viral, and Bacterial Sepsis using Multimodal Deep Learning. medRxiv Prepr 10: 1-ç.
- Brooks D., Polubothu P., Young D (2017) Sepsis caused by bloodstream infection in patients in the intensive care unit: the impact of inactive empiric antimicrobial therapy on outcome. J Hosp Infect 98: 369-374.
- Akilimali A., Oduoye MO., Balume A (2022) Antimicrobial use and resistance in Democratic Republic of Congo: Implications and recommendations; A mini review. Ann Med Surg 80: 104183.
- Evans L., Rhodes A., Alhazzani W (2021) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med; 47: 1181-1247.
- Boonstra JM., Märtson AG., Sandaradura I (2021) Optimization of fluconazole dosing for the prevention and treatment of invasive candidiasis based on the pharmacokinetics of fluconazole in critically ill patients. Antimicrob Agents Chemother 65: 1-11.
- Al-Ahmadey ZZ., Al-Ahmadi SS., Aljohani ED (2023) Candida Bloodstream Infection and Antifungal Susceptibility Over Three Years in a Single Center from Medinah, Saudi Arabia. Microbiol Res J Int 33: 1-7.
- Singer M., Deutschman CS., Seymour CW (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315: 801-810
- Singh G., Pitoyo CW., Aditianingsih D (2016) Risk factors for early invasive fungal disease in critically ill patients. Indian J Crit Care Med 20: 633-639.
- Singh T., Kashyap A., Ahluwalia G (2014) Epidemiology of fungal infections in critical care setting of a tertiary care teaching hospital in North India: A prospective surveillance study. J Clin Sci Res 3: 14.
- Rodriguez L., Bustamante B., Huaroto L (2017) A multi-centric study of Candida bloodstream infection in Lima-Callao, Peru: Species distribution, antifungal resistance and clinical outcomes. PLoS One 12: 1-12.
- Ragunathan L., Poongothai GK., Sinazer AR (2014) Phenotypic characterization and antifungal susceptibility pattern to fluconazole in Candida species isolated from vulvovaginal candidiasis in a tertiary care hospital. J Clin Diagnostic Res 8: 1-4.
- vom Steeg LG., Klein SL (2016) SeXX matters in infectious disease pathogenesis. PLoS Pathog 12: 1-6.
- Rayens E., Rayens MK., Norris KA (2022) Demographic and Socioeconomic Factors Associated with Fungal Infection Risk, United States, 2019. Emerg Infect Dis 28: 1955-1969.
- Ahn YH, Lee J., Oh DK (2023) Association between the timing of ICU admission and mortality in patients with hospital-onset sepsis: a nationwide prospective cohort study. J Intensive Care 11: 1-9.
- Driessen RGH., Heijnen NFL., Hulsewe RPMG (2021) Early ICU-mortality in sepsis-causes, influencing factors and variability in clinical judgement: a retrospective cohort study. Infect Dis (Auckl) 53: 61-68.
- Wójtowicz A., Tissot F., Lamoth F (2014) Polymorphisms in tumor necrosis factor-α increase susceptibility to intra-abdominal Candida infection in high-risk surgical ICU patients. Crit Care Med 42: 304-308.
- Rafat Z., Hashemi SJ., Ashrafi K (2020) Epidemiology, laboratory diagnosis and clinical aspects of fungal pulmonary infections in 384 patients hospitalized in pulmonary units in Guilan province, Iran. Iran J Microbiol 12: 353-363.
- Li Z., Lu G., Meng G (2019) Pathogenic fungal infection in the lung. Front Immunol 10: 1-20.
- Ruan H., Zhang Q., Zhang YP (2024) Unraveling the role of HIF-1α in sepsis: from pathophysiology to potential therapeutics—a narrative review. Crit Care 28: 1-15.
- Pavez N., Kattan E., Vera M (2020) Hypoxia-related parameters during septic shock resuscitation: Pathophysiological determinants and potential clinical implications. Ann Transl Med 2020; 8: 784-784.
- Metoto Mbengo JA., Noutakdie Tochie J., Ndom Ntock F., Nzouango JB KS, NgonoAteba G., et al. (2019) The Epidemiology, Therapeutic Patterns, Outcome, and Challenges in Managing Septic Shock in a Sub-Saharan African Intensive Care Unit: A Cross- Sectional Study. Hosp Pr Res 4: 117-121.
- Al Omar S., Alshraideh JA., Khassawneh B (2021) The Prevalence of Sepsis and Septic Shock in a Middle-Income Country: Experience of Two Tertiary Hospitals in Jordan. J Crit Intensive Care 12: 75-79.
- panelJin Zhu., Yanyan Dong., Pengda Liao., Xin Yin, Jianzhuo He LG (2024) Prognostic value of hemoglobin in patients with sepsis: A systematic review and meta-analysis. Hear Lung 64: 93-çç
- Zhong L., Zhong Y., Chen W (2024) Association between haemoglobin-to-red blood cell distribution width ratio at admission and all-cause mortality in adult patients with sepsis in an intensive care unit: a secondary analysis of the MIMIC-IV database. BMJ Open 14: 1-10.
- Rhodes A., Evans LE., Alhazzani W (2017) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43: 304-377
- Evaluation and management of severe sepsis and septic shock in adults (2021). Updat Wolters Kluwer. Gregory A: 1-22
- Bagshaw RAKBLGM (2015) Sepsis-Associated Acute Kidney Injury. Semin Nephrol 35: 2-11.
- Manrique-Caballero CL., Del Rio-Pertuz G., Gomez H (2021) Sepsis-Associated Acute Kidney Injury. Crit Care Clin 37: 279-301.
- Tsegaye EA., Teklu DS., Bonger ZT (2021) Bacterial and fungal profile, drug resistance pattern and associated factors of isolates recovered from blood samples of patients referred to Ethiopian Public Health Institute: cross-sectional study. BMC Infect Dis 21: 1-10.
- Lewis JM., Feasey NA., Rylance J (2019) Aetiology and outcomes of sepsis in adults in sub-Saharan Africa : a systematic review and meta-analysis. 23: 1-11.
- Delaloye J., Calandra T (2014) Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 5: 154-162.
- Dasgupta S., Das S., Chawan NS (2015) Nosocomial infections in the intensive care unit: Incidence, risk factors, outcome and associated pathogens in a public tertiary teaching hospital of Eastern India. Indian J Crit Care Med 19: 14-20.
- Zeng ZR., Tian G., Ding YH (2019) Surveillance study of the prevalence, species distribution, antifungal susceptibility, risk factors and mortality of invasive candidiasis in a tertiary teaching hospital in Southwest China. BMC Infect Dis 19: 1-12.
- Calandra T., Roberts JA., Antonelli M (2016) Diagnosis and management of invasive candidiasis in the ICU: An updated approach to an old enemy. Crit Care 20: 4-9.
- Dabas Y., Xess I., Pandey M., (2022) Epidemiology and Antifungal Susceptibility Patterns of Invasive Fungal Infections (IFIs) in India: A Prospective Observational Study. J Fungi 8.
- Seyoum E., Bitew A., Mihret A (2020) Distribution of Candida albicans and non- albicans Candida species isolated in different clinical samples and their in vitro antifungal suscetibity profile in Ethiopia. BMC Infect Dis 20: 1-9.
- Guinea J (2014) Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect 20: 5-10.
- Hernández-Pabón JC., Tabares B., Gil Ó (2024) Candida Non-albicans and Non-auris Causing Invasive Candidiasis in a Fourth-Level Hospital in Colombia: Epidemiology, Antifungal Susceptibility, and Genetic Diversity. J Fungi 10: 326.
- Meradji A., Ranque S., Bachtarzi F (2024) Incidence, Species Distribution, and Antifungal Susceptibility of Candida Bloodstream Infections in a Tertiary Algerian Hospital. Biol Life Sci Forum 31: 1-7.
- Guo LN., Yu SY., Xiao M (2020) Species distribution and antifungal susceptibility of invasive candidiasis: A 2016-2017 multicenter surveillance study in beijing, china. Infect Drug Resist 13: 2443-2452.
- Xiao Z., Wang Q., Zhu F (2019) Epidemiology, species distribution, antifungal susceptibility and mortality risk factors of candidemia among critically ill patients: A retrospective study from 2011 to 2017 in a teaching hospital in China. Antimicrob Resist Infect Control 8: 1-7.
- Soulountsi V., Schizodimos T., Kotoulas SC (2021) Deciphering the epidemiology of invasive candidiasis in the intensive care unit: is it possible? Infection 49: 1107-1131.
- Nascimento T., Guerreiro D., Toscano C (2024) Insights into Candida Colonization in Intensive Care Unit Patients: A Prospective Multicenter Study. Fungi 10: 1-13
- Riera FO., Caeiro JP., Angiolini SC (2022) Invasive Candidiasis: Update and Current Challenges in the Management of This Mycosis in South America. Antibiotics 2022; 11: 1-16.
- Al-Dorzi HM., Sakkijha H., Khan R (2020) Invasive Candidiasis in Critically Ill Patients: A Prospective Cohort Study in Two Tertiary Care Centers. J Intensive Care Med 35: 542-553.
- Badiee P., B.adali H., Boekhout T (2017) Antifungal susceptibility testing of Candida species isolated from the immunocompromised patients admitted to ten university hospitals in Iran: Comparison of colonizing and infecting isolates. BMC Infect Dis 2017; 17: 1-8. doi:10.1186/s12879-017-2825-7
- Bilal H., Shafiq M., Hou B (2022) Distribution and antifungal susceptibility pattern of Candida species from mainland China: A systematic analysis. Virulence 13: 1573-1589.
- Brunetti G., Navazio AS., Giuliani A (2019) Candida blood stream infections observed between 2011 and 2016 in a large Italian University Hospital: A time-based retrospective analysis on epidemiology, biofilm production, antifungal agents’ consumption and drug-susceptibility. PLoS One 14: 1-17.
- Bassetti M., Vena A., Bouza E (2020) Antifungal susceptibility testing in Candida, Aspergillus and Cryptococcus infections: are the MICs useful for clinicians? Clin Microbiol Infect 26: 1024-1033.
- Aldardeer NF., Albar H., Al-Attas M (2020) Antifungal resistance in patients with Candidaemia: A retrospective cohort study. BMC Infect Dis 20: 1-7.
- Ruiz-rodriguez M (2017) Sepsis: A Review of Advances in Management. 2393-2411.



