Organic and inorganic volatile compounds emitted from bacteria can be analyzed by headspace gas chromatography (HS-GC) if cultured in closed septum vials. Emission of hydrogen however has found less interest contrary to volatile organic compounds (VOCs) although it enables to check whether or not a sample is infected. However, if only a single compound such as hydrogen is monitored, a specific hydrogen sensor is suited as well and both techniques are described and compared on the example of an infected food and are also applied for veterinary and human medicine. Lyme disease was selected here as representative for diagnosis and therapy of other diseases caused by bacterial infection. Addition of standard or natural antibiotics to the samples in the vials enables to investigate the efficacy of their antibiotic effect. The electrical output of both HS-GC and the sensor approach enables digital data processing and documentation.
Bacteria; Hydrogen Emission; Hydrogen Sensor; Gas Chromatography; Antibiotics; Food; Lyme Disease; Food; Diagnosis; Therapy
Bacteria cultured in suitable culture media emit volatile compounds such as volatile organic compounds (VOC) which have found much interest, while inorganic emitted compounds such as hydrogen have found less attention [1], although hydrogen also provides interesting information about the activity of relevant bacteria. Hydrogen emitting bacteria comprises all classes of anaerobic, facultative anaerobic and micro aerobic bacteria. Analysis of emitted volatiles require closed containers and the technique of static headspace gas chromatography (HS-GC) therefore is an adequate analytical procedure. HS-GC is a powerful tool to separate complex mixtures of volatiles. If, however just a single compound should be taken as a key compound a specific sensor for that compound might be sufficient.
The sample to be investigated is placed into a headspace vial, which is sealed by screw cap closure. The 6 ml vials already contain 2.5 ml of sterile culture medium. The samples are inserted into the liquid culture medium. In case of a bitten tick the complete insect can be dipped into the liquid culture (cf. Figure.1) and also the ball of cotton wool from a cotton bud in case of a smear from the sample. The charged vials were then cultured at 35 °C in general for 2 days. The necessary time depends on the reproduction rate of the relevant bacteria. Some bacteria, e.g. spirochetes, were cultured usually for three days due to their slow reproduction rate. After then a 0.3 ml aliquot of the headspace gas is withdrawn by a gas tight syringe by piercing the septum of the vial and flushed against the H2-sensor (cf. Figure 1) and into the gas chromatograph.
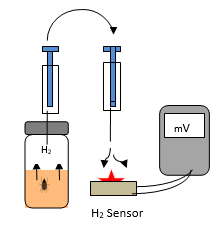
Figure 1: Emission of hydrogen from an infected tick and transfer from the headspace by a gas tight syringe to the hydrogen sensor with digital output (mV).
The presence of H2 is indicated by a red light (cf. Figure 1) at the hydrogen sensor but in addition to this optical response the electrical signal can also be traced by measuring the voltage output (mV). In the following examples the response from the H2-sensor either the positive (+) or the missing (-) H2 red light response is compared with the result of HS-GC in parallel. The resulting electrical output (mV) is included just as an example in Figure 2 only but not in the others, because the simple signal yes (red light) or no is sufficient to recognize whether or not a sample is infected while a digital result may be used for possible further research.
Bacterial Infection of Food
Food producing animals from poultry and fish farming are often contaminated by microbes and food, particularly offered in supermarkets is thus often contaminated by microbes. The H2 approach can not only be applied to control the integrity of the offered products to prevent the consumers from food-borne diseases but also to recognize any bacterial resistance because there is serious concern on the increasing number of multi resistant antibiotics. We have investigated a limited number of chemical and natural antibiotics, such as seed of cress, teasel, propolis, tree resin and some essential oils, with varying and not unambiguous success, depending on the sample type, the relevant bacteria, and some other parameters. From these compounds’ garlic (Allium sativum) and oil of cloves (Eugenia caryophyllus) have emerged as universally applicable natural antibiotics.
Figure 2. presents the results from a contaminated fish [2]. Trout from an aquatic farm was apparently contaminated as shown by the hydrogen emission, while another trout cached in a clean and cold river emitted no hydrogen (not shown here) and was thus not contaminated. This example was taken to compare the efficacy of a natural antibiotic clove oil with that of the standard doxycycline.
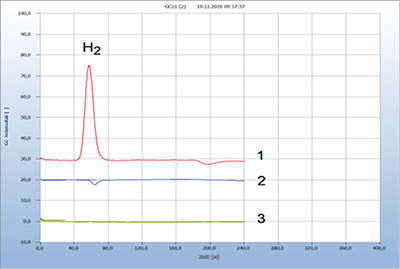
Figure 2: Efficacy of natural and chemical antibiotics.
1= infected trout (+, 280 mV), 2 = added streptomycin (-), 3 = added oil of cloves (-); GC column Silica gel.
Diagnosis of Bacterial Infection of Clinical Specimens and Antimicrobial Therapy
An important application is the search for effective antibiotics in both veterinary and human medicine. Figure 3 gives the result of searching for a suitable antibiotic in the case of a rabbit with inflamed nose and mouth [2, 3]. Unfortunately, this animal treated by the veterinary with amoxicillin died since apparently this antibiotic was the wrong drug, but treated with tetracycline, the rabbit probably would have survived.
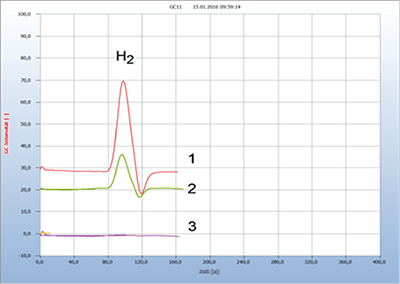
Figure 3: Antibiotic resistance against amoxicillin.
1 = rabbit with inflamed nose and mouth (+), 2 = added amoxicillin (+); 3 = added tetracycline (-). GC-column: Chromosorb 102
Diagnosis and Therapy of Lyme Disease Caused by Tick Infection of Humans
The foregoing examples show how essential oils can eliminate bacterial infections in various samples, but this method can also be applied in human medicine and tick-borne Lyme disease is taken here as representative for diagnosis and therapy of other diseases caused also by bacterial infections in humans. Tick-borne diseases, however, are not only caused by Borrelia burgdorferi because ticks are often contaminated with a complex mixture of co-infections. Borrelia bacteria are microaerophile and during the incubation time in a vial its metabolism change, starting with oxidation and emission of CO2, but as the oxygen concentration steadily declines due to the oxygen consumption the bacteria change to fermentation with emission of hydrogen because they can thrive in an atmosphere with reduced oxygen concentration compared to air.
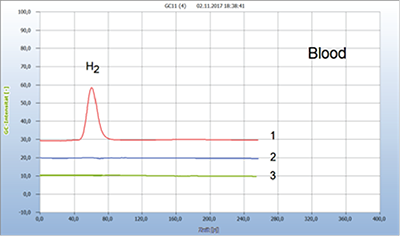
Figure 4: Borreliosis Therapy with Natural and Chemical Antibiotics
1 = blood (+) 2 = added garlic (-), 3 = added doxycycline (-) Since the H2-sensor approach allows to detect bacterial contamination it can also be used to check the eradication of such infections by chemical or natural antibiotics. If the hydrogen signal disappeared when such antibiotics are added to a blood sample in the headspace vial, that particular antibiotic is effective and if not, it is either inactive or the bacterium already resistant. It is thus an effective procedure to search for alternative antibiotics in case of antibiotic resistance. After garlic was found effective against contaminated food this drug was then applied against Borrelia in infected ticks and in the blood of patients suffering from tick-borne Lyme-disease [4, 5]. Figure 4 presents such an example. Blood aliquots from a patient suffering from borreliosis as shown by hydrogen emission of a blood sample have been treated in the septum vials with the standard doxycycline and alternatively with the natural antibiotic from a garlic capsule. Both the chemical and the natural antibiotic worked as well. The patient then was finally cured from borreliosis by the garlic therapy in because the patient suffered from serious side effects by the chemical antibiotic. The available standard techniques of diagnosis such as ELISA and Western Blot are not always sufficient reliable because these serological assays are indirect tests since they measure an antibody response to the infection, not the infection. All these indirect diagnostic techniques cannot control the progress of an antibiotic therapy and it is for these reasons why the recommendations on the necessary dosage and the adequate length of therapy vary widely from one week up to several months between many experts due to the uncertainty of the applied therapy. A too long extended antibiotic therapy may damage the micro biome and on the other hand a too early interruption is particularly dangerous. For all these reasons a reliable direct proof of pathogenic bacteria is needed, and such an approach is offered in this study with the H2 technique. It is the advantage of the method described that it allows complete control of the Borrelia infection just from the beginning with testing already the contaminated tick until the successful end of a suitable therapy. A systematic diagnosis and therapy may proceed according to the following procedure: A tick after having been removed from the body is analyzed immediately as to whether contaminated by Borrelia and the result is obtained already between 2 days and if the tick has been found contaminated, analysis of the blood shows whether the infection was transmitted to the blood, because not in all cases are the bacteria transmitted. If bacteria in the blood are found, a suitable therapy, either with chemical or natural antibiotics, should start immediately. The progress of the applied therapy is permanently controlled until its successfully end. Even after the therapy is finished, it is recommended to repeat this test regularly, particularly in the case of persistent borreliosis or in the case of re-growth with newly emerging health complaints. Only in the event of serious side- effects, which may often strike the patients, an alternative natural antibiotic can help, such as the described garlic therapy. However, other natural antibiotics may also work as well [6]. The garlic capsules used in this work have the advantage that no prescription is required and moreover they are free from unpleasant odours. The Swedish army has already applied such garlic capsules successfully as tick-repellents for military personnel [7].
The new method to prove the presence of bacteria in various samples by their hydrogen emission using either headspace gas chromatography or a specific hydrogen sensor has the unique advantage that the whole procedure is carried out in hermetically closed vials, this already beginning with sample preparation. When the samples are inserted into the culture broth, the headspace vials are capped with PTFE-lined septa and sealed. The septum remains tight, and several gas samples can be analyzed at given time intervals, thus enabling kinetic measurements. After analysis, the closed vials with their contents can be sterilized at the necessary high temperature and then safely discarded. Thus, the personnel in a lab never meet pathogens. Both techniques HS-GC, and the H2-sensor approach, may have different advantages and applications. HS-GC can be carried out with commercially available automated instruments and up to 100 samples can thus be processed unattended. It is therefore ideal for broad range screening purposes. The H2-sensor approach on the other hand is a simple technique and since such a H2-sensor is a very cheap. device it is feasible that this technique may be carried out even in the office of a physician or in a pharmacy since no strict safety requirements are afforded.
Both the technique of HS-GC and the H2-sensor approach are perfectly suited for quantitative analysis since both the resulting peak area of an HS-GC analysis and the electrical output (mV) of an H2-sensor lend itself for computer calculation. Any quantitative investigations, however, require standardization of all relevant parameters and also the preparation of certified calibration standards but these aspects may be a challenge for further research efforts.
Low-cost gas chromatograph GC-AK 11, Aug. Hedinger GmbH & Co KG, Germany. GC-columns: 0.8m x 6mm polyamide tube, packed with Silicagel 60/80mesh and 0.8m x 6mm polyamide tube, packed with Chromosorb 102, 60/80 mesh, room temperature, carrier gas ambient air. Headspace vials: 6 ml screw-capped from Restek, with PTFE-laminated butyl rubber septa. Culture medium: CASO-Bouillon tryptic soy broth acc EP+ USP 3080r-20p, Merck Life Science GmbH, Germany, composition: pancreatic digest of casein, 17g; papaic digest of soya bean meal, 3g; sodium chloride, 5g; dipotassium hydrogen phosphate, 2.5g; glucose monohydrate. Garlic capsules with pulverized garlic, Hirundo Products, FL-9493 Mauren 500mg/capsule with 1mg Allicin. Hydrogen sensor: Keyes MQ-8 Hydrogen gas sensor, Modul KS-046, fluxworkshop.
- Ichikawa, Yusuke.,Yamamoto, Haru., Hirano, Shin-ichi., Sato, Bunpei., Takefuji, Yoshiyasu., et al. (2023) The overlooked benefits of hydrogen-producing bacteria. Medical Gas Research 13: 108-111. [Crossref]
- Kolb BK., Riesterer L., Bier L., Widenhorn AM (2019) Proof of bacteria and the activity of chemical and natural antibiotics by headspace gas chromatography. Journal of Analytical Science and Technology 10: 1-9.
- Kolb B (2022) Monitoring the Emission of Hydrogen from Bacteria in Contaminated Food, Medical Specimens and in Human Blood, infected with Tick-borne Lyme Borreliosis by Gaschromatography and by a Specific Hydrogen Sensor and Examining the Efficacy of Natural Antibiotics. Am J Biomed Sci & Res 7. [Crossref]
- Kolb B., Riesterer L., Widenhorn AM., Bier L (2020) Monitoring of Hydrogen Emission from Bacteria in Food, Animals and in the Blood of Humans Suffering from Lyme Disease by A Specific Hydrogen Sensor. Antibiotics 9: 427. [Crossref]
- Kolb B. (2024) Determination of Emitted Hydrogen (H2) from Bacterial Cultures in Closed Septum Vials by Gas Chromatography (GC) and Specific Hydrogen Sensor Techniques. Research Perspectives of Microbiology and Biotechnology 3: 100.
- Feng J., Shi W., Miklossy J., Tauxe GM., McMeniman CJ (2018) Identification of Essential Oils with Strong Activity against Stationary Phase Borrelia burgdorferi. Antibiotics 7: 89. [Crossref]
- Stjernberg L., Berglung J (2000) Garlic as an insect repellent. JAMA 284; 829-883.



