Aims: Primary ectopic breast cancer (PEBC) is a rare and often misdiagnosed condition. Through the discussion of two clinical cases, we want to focus on clinical presentation, outcomes and treatment of PEBC, to lead clinicians to awareness and optimal management.
Methods: We present the case of a 47-year-old patient, with a 30 mm axillary mass, that was diagnosed as a PEBC (infiltrating lobular carcinoma, triple negative). The patient underwent systemic staging: diffuse metastatic bone lesions and leptomeningeal metastasis were found.
The second patient is a 73-year-old woman with personal history of right breast tumor. She came to our attention for a 9 mm left axillary mass, suspicious for a metastatic lymph node. A fine-needle cytology revealed the absence of lymphoid cells but the presence of atypical epithelial cells, as in a primary breast carcinoma. She was treated with local excision and sentinel node biopsy.
Results: The first patient presented with metastatic disease at the time of diagnosis and she deceased after three months from the diagnosis, despite systemic chemotherapy. The diagnosis was performed at an early stage in the second patient. She underwent surgery, complementary endocrine therapy and radiotherapy. She has no evident disease after two years from surgery.
Conclusion: Primary ectopic breast cancer is a rare clinical entity, often misdiagnosed or diagnosed with a long delay. The treatment of PEBC is analogous to that of orthotopic breast cancer, but we strongly recommend to approach the patient with a multidisciplinary team to provide the best staging workout and therapies.
Ectopic breast tissue; Ectopic breast cancer; Axillary masses; Multidisciplinary management
Ectopic Breast Tissue (EBT) is the most common congenital breast abnormality [1]. The occurrence of EBT ranges from 0,6% to 6% in the general population. Multiple theories have been proposed to explain the occurrence of accessory breast. These include Darwin’s theory which stated that “traits which have disappeared generations before, can reappear”; Pfeifer’s theory of metaplasia of sweat glands or modified sweat glands; Hughes’s theory of random migration of primordial breast cells away from the mammary crest, and Schultz’s theory of displacement of milk lines, laterally or caudally [2-5]. Despite morphological differences, EBT presents characteristics analogous to orthotopic breast tissue in terms of function and pathological degeneration. EBT comes in two forms: supernumerary and aberrant [6]. A supernumerary breast consists of a ductal system communicating with the overlying skin, usually located along the ‘‘milk line’’, which extends from the axilla to the groin. It frequently responds to hormonal stimulation and undergoes physiologic changes as a complete functioning breast [7], is subject to the same diseases and alterations, whether benign or malignant, that affect orthotopic breasts [8,9]. Two varieties of supernumerary breast should be distinguished: polymastia and polythelia. Polymastia (less than 1% of the population) is an accessory gland that occur as a consequence of the mammary ridge failing to regress during embryonic development. The gland may be found in association with an areola-nipple complex. Polythelia (about 1,4% of the population) presents itself in the form of an areola and/or a nipple lacking glandular tissue [10]. Aberrant breast consists of an isolated fragment of glandular tissue located beyond the periphery of orthotopic breasts; it is characterized by an unorganized secretory system without any connection between the inside and the outside. The axilla is most frequently involved (70%) and the glandular tissue is located in the subcutaneous tissue and deep dermis [11]. Another 20% occurs in thoracic or abdominal portion of milk line, often on the left side of the body just below the inframammary crease [12]. EBT formation has also been found outside the galactic band that is in the face, posterior neck, midback, buttock, vulva, flank, hip, posterior and/or lateral thigh, shoulder and upper extremities [11]. This condition has also been associated with other congenital anomalies (nervous, cardiovascular, gastroenteric, skeletal, and, particularly, renal abnormalities) [8,13-15]. Data in the literature concerning the probability of malignant degeneration of EBT, and its management are scanty and controversial [10]. All articles regarding Primary Ectopic Breast Cancer (PEBC) are case reports and historical reviews. No systematic reviews or clinical trials are available. Given the rarity of this clinical condition, no specific guidelines are available in literature. Thus, early diagnosis and optimal management are still challenging for clinicians. The aim of this article is to describe two cases of breast cancer involving an EBT and to review the most recent literature.
Patient 1: A 47-year-old Caucasian woman with familiar history of breast cancer, performing regular annual clinical examination and mammography as part of the screening for SHBC patients. At clinical examination, a 30 mm hard mass was detected in the left axilla, with no evident communication with the skin nor any structure referable to a nipple-areola-like complex (aberrant tissue). Imaging with mammography and high definition ultrasound revealed a hypo-echoic 25 mm mass, suspicious for a neoplasm on ectopic mammary gland. The radiological diagnosis was completed with breast MRI, which confirmed a 48×45 mm mass and an enlarged 20 mm lymph node. A core biopsy of the axillary suspicious mass was performed, and resulted in an infiltrative lobular carcinoma, triple-negative and MIB-1: 12% (Figure 1). In the orthotopic left mammary gland two more nodular masses were found (diagnosed as C4 at a fine-needle aspiration cytology) and a 14 mm lymph node was visible in the omolateral axilla. An interdisciplinary team approach recommended systemic staging with a thoraco-abdominal CT scan, that showed diffuse metastatic bone lesions (sternum, hip bones, dorsal and lumbar vertebrae, femoral bone), confirmed by a bone scintigraphy.
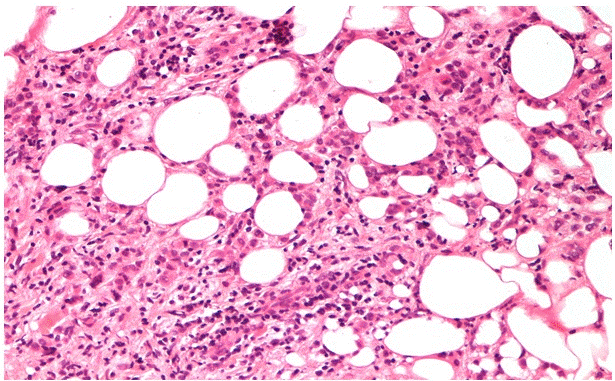
Figure 1. H&E sections of lobular carcinoma in Patient 1
Patient 2: A 73-year-old Caucasian woman with familiar history of breast cancer (a maternal aunt and three maternal cousins at the age of 33, 70 and 75 with negative BRCA mutation test). She underwent right mastectomy with reconstructive surgery and axillary lymphadenectomy two years before for a triple negative ductal infiltrative carcinoma of the breast. Surgery was followed by adjuvant chemotherapy with epirubicin and cyclophosphamide for four cycles and weekly paclitaxel for 12 administrations. Follow up was negative, until a 9 mm nodular lesion was detected by ultrasound in the left axillary cavity. The first clinical suspicion was for a metastatic lymph node even if the mass was in a subcutaneous position. A fine-needle aspiration cytology was performed and revealed the presence of atypical epithelial cells, but no lymphoid cells were detectable. The diagnostic suspicion thus turned for a neoplasm of an ectopic mammary gland, in its aberrant form. A breast MRI and a systemic re-staging with thoraco-abdominal CT scan were done and did not show any distant metastasis.
Patient 1: The patient underwent systemic chemotherapy with FEC (fluorouracil, epirubicin and cyclophosphamide) for four cycles and simultaneous zoledronic acid, followed by Taxotere every three weeks. She also received radiation therapy (3 Gy x 10 fractions) on the acetabulo-femoral joint and on ischio-pubic hip bone. At the end of the treatment, a local restaging with breast MRI was performed and showed a reduction of the axillary mass (39×20 mm). The two smaller nodular masses were no longer detectable. The bone scintigraphy showed a downsizing of the metastatic bone lesions and no new metastatic lesions were visible on the thoraco-abdominal CT scan.
Seventeen days after the end of chemotherapy the patient presented nausea, vomiting, dizziness and vertigo. A brain CT scan without contrast medium was performed and did not show any brain lesion. The patient was treated with corticosteroid therapy with transitory reduction of symptoms. A few days after, temporal-spatial disorientation and headache appeared. The patient underwent a brain MRI with contrast medium, which showed the presence of leptomeningeal metastasis. The examination of the cerebrospinal fluid confirmed the diagnosis, showing multiple neoplastic epithelial cells. A supportive therapy was administered until the patient deceased, one month later.
Patient 2: An interdisciplinary team approach recommended wide local excision and sentinel node biopsy. The final pathological report revealed a grade 1 papillary carcinoma of the breast (Figure 2), with ER 98%, PgR 98%, Her-2 negative and MIB-1: 4%. Sentinel node was negative. The patient received both adjuvant endocrine therapy with Tamoxifen and external beam radiotherapy to whole residual breast and ipsilateral axillary region, until completing 50 Gy. She is in good clinical conditions with no evident disease after two years from surgery.
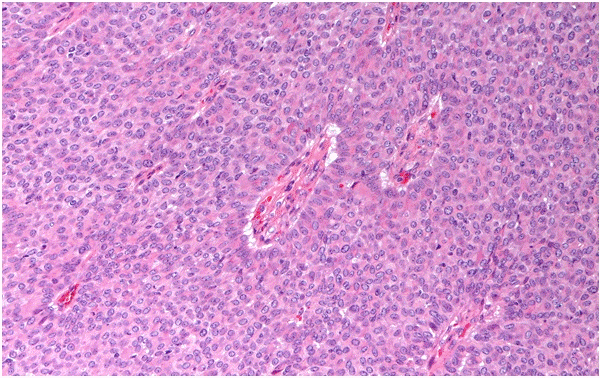
Figure 2. H&E sections of papillary carcinoma in Patient 2
Both case reports present an opportunity to discuss clinical presentation, outcome and treatment of PEBC. Despite morphological differences EBT, whether supernumerary or aberrant, present characteristics analogous to orthotopic tissue in terms of function and pathological degeneration, including carcinoma [16,17]. PEBC is uncommon, accounting for 0,2 to 0,6 % of all breast cancers [12], but it has been reported in 60% to 70% of all forms of ectopic breast tumor. More frequently ectopic breast cancer is found in aberrant breast tissue, as it was in both the cases we presented. Clinical diagnosis is more challenging in aberrant tissue, because no visible anatomical structure (nipple-areola complex) suggests the underlying presence of breast tissue, as it is in complete supernumerary breast. Only a minority of patients develop breast cancer on a complete supernumerary breast. Some researchers consider malignant degeneration of EBT to be highly probable. In particular, aberrant breast tissue is reportedly more prone to malignant transformation than orthotopic or ectopic breast tissue [18,19], due to the stagnation in the ductal lumens which is considered a promoting factor for the development of malignancy [6]. It is no clear if PEBC has a race-prevalence as for BC. Some papers describe the highest incidence among Japanese and the lowest among Caucasians [11]. Familiar transmission account 10% of cases, as in the second case report reported above [12]. PEBC seems to affect people at a younger age compared to BC. However, PEBC shows a 50 % peak of incidence between 40 and 45 years and a median age at diagnosis of 51 years [11]. There is a general belief that the risk factors for BC apply also to ectopic tissue. Clinical diagnosis is often delayed if not systematically considered in the differential diagnosis due to unfamiliarity with the condition, even in patients that regularly perform examinations for breast cancer familiar predisposition or in patients that already have a personal clinical history of breast cancer. The estimated delay in diagnosis is around 3.3 years. The presence of a subcutaneous mass along the milk line with clinical features of palpable mass, skin thickening, tenderness, or edema [20], should provoke a high suspicion for PEBC and this disease should be ruled out first [21]. Ultrasound is the first additional step: the presence of a hypo-echoic, not well-defined heterogeneous mass, without signs of inflammation, is suspicious [22]. Mammography may add further information even if micro calcifications of EBT are difficult to delineate [23,24]. The differential diagnosis of an axillary nodular mass must always include other subcutaneous masses such as fibroadenoma, lipoma, abscess, sebaceous cyst, hydradenitis or follicular cyst, haematoma, seroma, foreign body and lymphadenopathy associated with benign (mastitis, breast abscess, trauma, infection to upper extremity, tularemia, cat scratch disease, TBC, actinomicosis, etc) or malignant disease [19,25]. Particularly, PEBC should be included in the differential diagnosis over metastatic nodes from chest and breast neoplasms, skin cancer, Hodgkin disease, adnexal malignant or soft tissue tumor. Therefore, in case of a unilateral axillary mass, breast, upper extremity and chest wall must always be accurately checked. In case of a suspicious lesion, Fine-Needle Aspiration Biopsy (FNAB)/core biopsy of the axillary lump should be performed to harvest cells/tissue for histologic diagnosis [26]. If a PEBC is diagnosed, the regular work-up guidelines for BC should be followed. MRI (Magnetic Resonance Imaging) might be useful in defining the extension of the PEBC and its relationship with normal breast tissue before surgery [27] and for the best differential diagnosis between PEBC and occult primary breast cancer. Invasive ductal carcinoma Not Otherwise Specified (NOS) is the most common histotype both in orthotopic (40-75%) and ectopic breast tissue (72%) [9,25,28], followed by invasive lobular carcinoma and medullary carcinoma (12%). Several pathological and immunohystochimic features can help to define the diagnosis: (1) Histologic pattern of a primary breast carcinoma in situ, (2) Presence of normal breast tissue surrounding the tumor, (3) Specific immunohistology for BC such as Estrogen and/or Progesteron receptors, and common breast markers such as Gross Cystic Disease Fluid Protein (GCDFP)-15. Although Her2/Neu, CEA and glandular keratins can be expressed in BC, they do not discriminate between a PEBC and a skin adnexal tumor, because they are not specific for the breast. Axillary node metastasis should be excluded too. The lymphatic spread of PEBC is most likely towards the homolateral axillary nodes and from there towards the supraclavicular nodes, because this is the normal lymphatic drainage of the subcutaneous and cutaneous tissues of the armpit. Whether to consider the internal mammary lymph nodes as distant metastasis instead of loco-regional involvement is still discussed and it affects systemic staging (M1 versus N1 or N2). When PEBC has been diagnosed, it is classified according to the TNM staging system used for primary breast carcinoma [24] and it should be treated in the same way as orthotopic breast disease. As we described in our clinical reports, both oncological and surgical approaches must be considered as primary treatment, depending on the stage, the performance status of the patient and the histopathological features of the tumor. The role of Sentinel Node Biopsy (SNB) for PEBC of the axilla is not clear. The general trend has been to perform axillary clearance in all patients. However, in our second case report, after multidisciplinary discussion, the patient underwent sentinel node biopsy just to avoid a needless axillary clearance. Prognosis of accessory breast carcinoma is difficult to ascertain due to its rarity. Poor outcomes compared to native breast cancer have been reported, which may relate to delayed diagnosis often until clinical symptoms are present [29]. A higher incidence of lymph node involvement compared with primary breast carcinoma in the native breast may also contribute to different outcomes [11]. The location of primary breast carcinoma in axillary breast tissue, closer to axillary lymph nodes, has been linked theoretically to earlier nodal metastatic disease [30,31], but for example in our two cases we found early distant metastasis in one case and no metastasis in the lymph node in the other. Although speculative, this may be explained by the fact that the growing of metastases is related to biologic properties of the tumor rather than to the anatomic properties of the localization [11]. No specific treatment protocol is available in the literature for axillary PEBC and, furthermore, there is no consensus on whether to excise the EBT prophylactically, to prevent the malignancy [32,33]. Traditionally, the surgical approach of PEBC was more aggressive, justified by the limited knowledge of its biological behavior and by the paucity of adjuvant treatments available: surgeons performed either a local tumorectomy or an ipsilateral prophylactic mastectomy. Cogswell and Czerny concluded that local excision combined with axillary dissection was the surgical procedure of choice [34]. Evans and Guyton suggested that radical or modified radical surgery offered no advantage over local excision combined with axillary dissection or radiation with respect to outcomes [35]. Nowadays, the conservative approach (local excision on primary tumor and complementary radiotherapy), is always considered as the first surgical choice when feasible. This behavior is in line with the one that is generally adopted in orthotopic breast treatment. Adjuvant treatments that had been found to be effective for BC were successfully applied to PEBC as well. Hormonal therapy, when appropriate, was an integral part of the treatment, as well. Adjuvant radiotherapy should be considered to optimize loco-regional control. A review of the literature offers no formal guidance on RT dose, fractionation, and treatment fields in this setting. Treatment fields have ranged from incorporating the tumor bed alone to inclusion of the ipsilateral uninvolved breast, axilla and supraclavicular fossa, even in the absence of nodal metastases [36]. Since there is no clear benefit to irradiate homo-lateral anatomic breast it is not performed systematically. Some authors report that it must be considered in cases of uncertainty between an axillary EBT or axillary metastasis from unknown primary breast cancer [37]. A careful follow-up is essential because of the limited knowledge of this tumor.
Primary ectopic breast cancer is a rare clinical entity often misdiagnosed or diagnosed with a long delay, leading to a poorer prognosis as compared to native breast cancer. The problem stems in part from a general lack of awareness that ectopic/accessory breast tissues have potentially dangerous implications, often underestimated by patients and physicians. We recommend to regularly include the possible sites of ectopic breast tissue in clinical examination and in the presence of an abnormal mass, always consider malignant tumor on ectopic breast in the differential diagnosis. Equivocal lesions, including microcalcification, should be considered suspicious until otherwise proven, followed up with close surveillance and/or candidate for diagnostic assessment. As we described in our cases, the treatment of PEBC is analogous to that of orthotopic breast cancer, but we strongly recommend to approach the patient with a multidisciplinary team to provide the best staging workout and therapies.
- Famá F, Cicciú M, Sindoni A, Scarfó P, Pollicino A, et al. (2016) Prevalence of ectopic breast tissue and tumour: a 20 year-single center experience. Clin Breast Cancer. 16: 107-112. [Crossref]
- Darwin C (1892) The descent of man and selection in relation to sex. New York: Appleton and Co. P: 36-37. [Crossref]
- Hughes ES (1950) The development of the mammary gland: Arris and Gale lecture, delivered at the Royal College of Surgeons of England on 25th October, 1949. Ann R Coll Surg Engl. 6: 99-119. [Crossref]
- Pfeifer JD, Barr RJ, Wick MR (1999) Ectopic breast tissue and breast-like sweat gland metaplasias: an overlapping spectrum of lesions. J Cutan Patholo. 26: 190-196. [Crossref]
- Schultz A (1933) Pathologische anatomic der brustdruse. In: Handbuch der speziellen Pathologischen Anatomie and Histologie. Berlin: Julius Springer. [Crossref]
- Williams WR (1891) Polymastism, with special reference to mammae erraticae and the development of neoplasms from supernumerary mammary structures. J Anat Physiol 25: 225-255. [Crossref]
- Nakao A, Saito S, Inoue F, Notohara K, Tanaka N (1998) Ectopic breast cancer: a case report and review of the Japanese literature. Anticancer Res. 18: 3737-3740. [Crossref]
- Senatore G, Zanotti S, Cambrini P, Montroni I, Pellegrini A, et al. (2010) Ectopic breast fibroadenoma: a case report. G Chir. 31: 96-99. [Crossref]
- Giron G, Friedman I, Feldman S (2004) Lobular carcinoma in ectopic axillary breast tissue. Am Surg. 70: 312-315. [Crossref]
- Francone E, Nathan MJ, Murelli F, Bruno MS, Traverso E, et al. (2013) Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 37: 746-749. [Crossref]
- Visconti G, Eltahir Y, Van Ginkel RJ, Bart J, Werker PM (2011) Approach and management of primary ectopic breast carcinoma in the axilla: where are we? A comprehensive historical literature review. J Plast Reconstr Aesthet Surg. 64: 1-11. [Crossref]
- Fachinetti A, Chiappa C, Arlant V, Lavazza M, Liu X, et al. (2018) Metachronous bilateral ectopic breast carcinoma: a case report. Glan Surg. 7: 234-238. [Crossref]
- Merlob P (2003) Congenital malformations and developmental changes of the breast: a neonatological view. J Pediatr Endocrinol Metab. 16: 471-485. [Crossref]
- Yuksel S, Coban YK (2011) Polymastia with diastometamyelia: Case Report. J Innu Univ Med Faculty. 18: 47-49. [Crossref]
- Zannolli R, Mostardini R, Matera M, Pucci L, Gelb BD, et al. (2000) Char syndrome: an additional family with polythelia, a new finding. Am J Med Genet. 95: 201-203. [Crossref]
- Chan N, Penswick J, Labelle E, Driman DK (2007) Ectopic breast tissue presenting as an anal polyp. Can J Surg. 50: E23-E24. [Crossref]
- Cutler M (1962) Tumors of the breast. Pitman Medical, London. [Crossref]
- Marshall M, Moynihan J, Frost A, Evans SR (1994) Ectopic breast cancer: a case report and literature review. Surg Oncol. 3: 295-304. [Crossref]
- Ghosn SH, Khatri KA, Bhawan J (2007), Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. Cutan Pathol. 34: 9-13. [Crossref]
- Pardo M, Silva F, Jiménez PP, Karmelic M (2001) Mammary carcinoma in ectopic breast tissue. A case report. Rev Med Chil. 129: 663-665. [Crossref]
- Tjalma WA, Senten LL (2006) The management of ectopic breast cancer: case report. Eur J Gynaecol Oncol. 27:414-416. [Crossref]
- Vignal P (2005) Sonographic appearance of a carcinoma developed in ectopic axillary breast tissue. J Clin Ultrasound. 33: 468-470. [Crossref]
- Muttarak M, Chaiwun B, Peh WC (2004) Role of mammography in diagnosis of axillary abnormalities in women with normal breast examination. Australas Radiol. 48: 306-310. [Crossref]
- Lee J, Jung JH, Kim WW, Hwang SO, Kange JG, et al. (2014) Ductal Carcinoma Arising from Ectopic Breast Tissue Following Microcalcification Observed on Screening Mammography: A Case report and review of the literature. J Breast Cancer. 17: 393-396. [Crossref]
- Nihon-Yanagi Y, Ueda T, Kameda N, Okazumi S (2011) A case of ectopic breast cancer with a literature review. Surg Oncol. 20: 35-42. [Crossref]
- Velanovich V (1995) Fine needle aspiration cytology in the diagnosis and management of ectopic breast tissue. Am Surg. 61: 277-278. [Crossref]
- Capobianco G, Spaliviero B, Dessole S, Rocca PC, Cherchi PL, et al. (2007) Lymph node axillary metastasis from occult controlateral infiltrating lobular carcinoma arising in accessory breast: MRI diagnosis. Breast J. 13: 305-307. [Crossref]
- Teke Z, Kabay B, Akbulut M, Erdem E (2008) Primary infiltrating ductal carcinoma arising in aberrant breast tissue of the axilla: a rare entity: report of a case. Tumori. 94: 577-583. [Crossref]
- Yerra L, Karnad AB, Votaw ML (1997) Primary breast cancer in aberrant breast tissue in the axilla. South Med J. 90: 661-662. [Crossref]
- Routiot T, Marchal C, Verhaeghe JL, Depardieu C, Netter E, et al. (1998) Breast carcinoma located in ectopic breast tissue: a case report and review of the literature. Oncol Rep. 5: 413-417. [Crossref]
- Copeland MM, Geschickter CF (1950) Diagnosis and treatment of premalignant lesions of the breast. Surg Clin North Am. 30: 1717-1741. [Crossref]
- Grossl NA (2000) Supernumerary breast tissue: historical perspectives and clinical features. South Med J. 93: 29-32. [Crossref]
- Emsen IM (2006) Treatment with ultrasound-assisted liposuction of accessory axillary breast tissues. Aesthetic Plast Surg. 30: 251-252. [Crossref]
- Cogswell HD, Czerny EW (1961) Carcinoma of aberrant breast of the axilla. Am Surg 27: 388-390. [Crossref]
- Evans DM, Guyton DP (1995) Carcinoma of the axillary breast. J Surg Oncol. 59: 190-195. [Crossref]
- Hallam S, Aggarwal A, Predolac D, Cunnick G, Ashford R (2013) Primary ectopic breast carcinoma in a supernumerary breast arising in the anterior chest wall: a case report and review of the literature. J Surg Case Rep. 2013: rjt107. [Crossref]
- Soares A, Gonçalves J, Azevedo I, Pereira HG (2013) Lobular ectopic breast carcinoma: a case-report. Rep Pract Oncol Radiother. 18: 189-191. [Crossref]


