Background: Acute appendicitis is one of the most common abdominal pains in children. Perforated appendicitis can result in a variety of potentially serious complications. The aim of this study was to evaluate the value of procalcitonin (PCT) and C-reactive protein (CRP) as predictors of appendiceal perforation in children.
Materials and methods: We identified 201 children (75 female; 126 male, mean ages, 7.6 y) with histologically confirmed acute appendicitis after appendectomy between January 2015 and January 2018. Preoperative laboratory data such as CRP, white blood cell (WBC), PCT and part of postoperative histologic results were analyzed retrospectively. We also performed a multivariate logistic regression model to determine patient's age and laboratory tests associated with perforated appendicitis.
Results: Mean plasma PCT level of all patients was 1.98 ng/mL (5.73 SD; range, 0.02–64.77 ng/mL; median, 0.18ng/mL). Children with appendiceal perforation had a mean PCT level of 6.26 ng/mL (9.39 SD; range, 0.2–64.77 ng/mL; median, 3.10 ng/mL), which was significantly higher than those with non-perforated children (P=0.001). Mean CRP values were 60.24 mg/L (55.02 SD, range, 1.0-260 mg/L; median, 44.0 mg/L) in all patients, whereas they were significantly higher in perforated group (mean, 114.53 mg/L, 53.88 SD, range, 30-260 mg/L) compared with non-perforated children (P = 0.001). Multivariate logistic analysis revealed that both PCT and CRP were associated with perforation (P=0.001). Area under the receiver operating characteristic curve (ROC) of PCT for discriminating acute perforated appendicitis from non-perforated groups was larger than that of WBC and CRP.
Conclusions: Both PCT and CRP are useful markers, and PCT is better than CRP for the diagnosis of perforated appendicitis in children.
procalcitonin; C-reactive protein; appendiceal perforation; diagnosis; children
Acute appendicitis is one of the most common abdominal pains in children [1]. The diagnosis of acute appendicitis in children is a challenge as many children with acute appendicitis do not have typical clinical manifestation [2-3]. Furthermore, younger children, especially infants, usually cannot describe pain and many of them just have some nonspecific signs such as irritability, anorexia and lethargy [4]. The initial misdiagnosis rate in children is very high, ranging from 28% to 57% for older children and nearly 100% for those two years or younger [5]. The delay in the diagnosis of an acute appendicitis can result in increased risk of perforation. The rate of perforated appendicitis varies from 5% to 75% and can even reach 100% for those who are less than one year of age [6]. Perforated appendicitis can result in a variety of potentially serious complications such as bacterial peritonitis, small bowel obstruction, and abdominal abscess formation. This leads to a longer hospital stay and higher mortality rate [7]. Therefore, it is very important for pediatric surgeons to improve preoperative diagnosis rate of perforated appendicitis accurately. Several previous studies have evaluated C-reactive Protein (CRP), Procalcitonin (PCT), and White Blood Count (WBC) for predicting the severity of acute appendicitis [8-9]. In this study we performed a retrospective chart review of 201 children with histologically confirmed acute appendicitis in whom biochemical markers were routinely tested before surgery. We expected to evaluate the role of C-reactive protein, Procalcitonin (PCT), and White Blood Count (WBC) in predicting an appendix perforation.
All children less than 15 years old admitted between January 2015 and January 2018 were screened in the study. Patients were included in the study if they had appendectomies (laparoscopic or open) and histopathological findings consistent with acute appendicitis. However, patients were deemed ineligible if they had appendiceal abscess or under ant biotherapy in the study. The initial diagnosis was based on the patient's symptoms and abdominal examination and it was confirmed by pathological report after surgery in all of them. The preliminary diagnosis of appendiceal perforation was made by the surgeon on the basis of macroscopy appearance and all cases that were identified as having a macroscopic perforation were sent for pathological confirmation. After a retrospective chart review, 201 consecutive patients were enrolled in the study. Routine preoperative C-reactive protein, procalcitonin, and white blood count for patients undergoing appendectomy were collected and analyzed. The normal range of procalcitonin levels is between 0 and 0.46 ng/ml. Normal value of WBC and CRP were defined as 4.0/nL to 12.0/nL and less than 8.0 mg/L, respectively.
Statistical analysis
SPSS Version 20.0 was applied for statistical analysis. Mean values and SD were calculated for WBC, CRP, and PCT levels. The data distribution was assessed by the Kolmogorov-Smirnov test. For normal distributed data, Student’s t test was used to compare the perforated and non-perforated groups. For non-normal data distribution, the Kruskal–Wallis test was used instead. A multivariate logistic regression analysis was performed to identify variables that were associated with perforated appendicitis. The independent variables of interest were age, PCT, WBC, and CRP. In addition, calculation of sensitivity, specificity, negative predictive value, and positive predictive value of PCT, WBC, and CRP as predictors for appendiceal perforation was included in data analysis. Statistical significance was set at the 5% level (2-sided).
A total of 201 children (75 female; 126 male; median age, 7.6±3.0 SD y; range, 2-15y) with histologically confirmed acute appendicitis were included in the study. The mean values of PCT, WBC, and CRP for these patients are listed in Table 1.The mean PCT level of all patients in the present study was 1.98 ng/mL(5.73 SD; range, 0.02–64.77ng/mL; median, 0.18ng/mL). The mean PCT level for patients in perforated children was 6.26 ng/mL (9.39 SD; range, 0.2–64.77ng/mL; median, 3.10ng/mL), which was significantly higher when compared with the non-perforated ones (P=0.001). Of the potential relevant variables (age, WBC, CRP, and PCT), logistic regression analysis indicated CRP and PCT had independent association with increasing perforation rates (P=0.001; Table 4).
Receiver Operating Characteristic Curve (ROC) analysis for PCT, WBC, and CRP shown that PCT had the largest area under receiver operating characteristic curve (0.97) compared with WBC (0.54) and CRP (0.89) (Figure 1). The optimal ratio of sensitivity (0.93) and specificity (0.93) was calculated when the PCT level was greater than 0.49 ng/mL regarding perforated appendiceal (Table 3). A cutoff for PCT of greater than 0.46ng/mL had 93% sensitivity and a negative predictive value of 93% for appendiceal perforation. Specificity of PCT for the preoperative diagnosis of appendiceal perforation was the highest (0.92) and PCT still had an acceptable sensitivity for predicting appendiceal perforation compared with WBC and CRP. Details of sensitivity, specificity and predictive values are listed in Table 2.
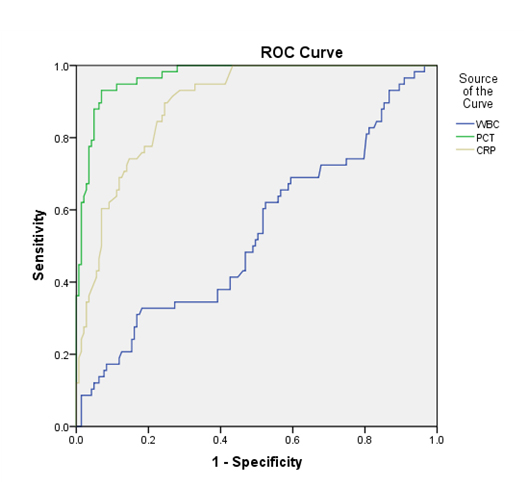
Figure 1. Receiver operating characteristic curve analysis for PCT, WBC, and CRP showed PCT had the largest area under receiver operating characteristic curve (0.97) compared with WBC (0.54) and CRP (0.89)
Table 1. Details of laboratory values in perforated and non-perforated children in the present study
Group |
CRP (mg/L) |
WBC (×109) |
PCT (ng/ml) |
Perforated children-58 |
114.53 ± 53.88 |
15.61 ± 6.20 |
6.26 ± 9.39 |
Non-perforated children (143) |
38.22 ± 37.50 |
14.55 ± 5.71 |
0.25 ± 0.59 |
P value |
0.001 |
0.354 |
0.001 |
Table 2. Sensitivity, specificity, positive predictive value, and negative predictive value for WBC, CRP, and Procalcitonin (PCT)
Laboratory value |
sensitivity |
specificity |
Positive predictive value |
Negative predictive value |
WBC>12/nl |
0.69 |
0.35 |
0.51 |
0.53 |
CRP>8mg/L |
1 |
0.25 |
0.57 |
1 |
PCT>0.46ng/ml |
0.93 |
0.92 |
0.92 |
0.93 |
The mean value of WBC was 14.8/nL (5.8 SD; range, 3.3-38.8/nL) whereas patients in perforated group had a mean of 15.6/nL (6.2 SD; range, 4.7-38.8/nL) with no statistical difference compared with patients in the non-perforated group (P=0.354). Mean CRP values were 60.24 mg/L (55.02 SD, range, 1.0-260 mg/L; median, 44.0mg/L) in all patients, whereas they were significantly higher in perforated group (mean, 114.53 mg/L, 53.88 SD, range, 30-260 mg/L) compared with non-perforated children (P = 0.001) (Table 1). ROC analysis shown that the highest sum of sensitivity and specificity for CRP was 55.0mg/L. A cutoff for CRP of greater than 8 mg/L had 100% sensitivity and 25% specificity for appendiceal perforation. The specificity was lower in predicting appendiceal perforation compared with PCT (Table 2).
Table 3. The highest sum of sensitivity and specificity for WBC, CRP and Procalcitonin(PCT)
Laboratory value |
sensitivity |
specificity |
Serum level |
WBC |
0.44 |
0.71 |
15.38 |
CRP |
0.89 |
0.75 |
55 |
PCT |
0.93 |
0.93 |
0.49 |
Table 4. Multivariate analysis of age and laboratory tests in relation to perforated appendix rates
Factor |
OR(95%CI) |
p |
Age |
0.99(0.81-1.21) |
0.92 |
CRP |
1.02(1.01-1.04) |
0.001 |
PCT |
3.20(1.75-5.85) |
0.001 |
WBC |
0.95(0.86-1.04) |
0.27 |
Acute appendicitis is a common cause of acute abdomen and requires surgical treatment, while delayed diagnosis can lead to perforation. The reported overall perforated rate of appendicitis is 18.3% and 34.0%, but this rises remarkably in children or older patients. Appendiceal perforation is associated with plenty of postoperative complications and higher mortality compared to appendicitis without perforation [7]. Appendectomy for perforated appendicitis is still a topic of debate because of the presence of severe inflammation and serious adherence of the surrounding structures. Emergency surgery is strongly recommended for non-perforated appendicitis while nonsurgical treatment may have a lower complication rate for perforated appendicitis [10]. Therefore, accurate preoperative diagnosis of a perforated appendicitis becomes very important. Physical examination such as diffuse peritonitis and board-like rigidity of the abdomen are helpful for the preoperative diagnosis of perforation, but the definitive diagnosis of perforated appendicitis still requires surgery and histological diagnosis. Although laboratory testing such as WBC and CRP are useful makers for diagnosing the appendicitis, their sensitively and specificity in predicting appendiceal perforation are not well studied.
Procalcitonin is constitutively secreted by the K cells of the lungs and the C cells of the thyroid gland, and PCT level always increases with severity of infection in children [11-12]. Unlike CRP and some other biomarkers, the PCT level does not raise with viral infection or sterile inflammation in patients [13]. Therefore, PCT can be used as an important marker of bacterial infection, such as sepsis, wound infection and acute appendicitis. Several studies have shown PCT was an excellent marker of severe bacterial infection. For example, the level of plasma PCT is significantly higher in the infection of renal parenchyma than lower urinary tract infection [14]. Besides, because of its high sensitivity and specificity, PCT has even been applied to predict the surgical wound infection after heart surgery [15]. Although PCT has a lower diagnostic value for diagnosing acute appendicitis compared with other markers such as CRP or WBC [16-18], several studies have suggested that PCT was a useful marker for predicting complicated appendicitis [18-19]. Yu et al. conducted a systematic review on the diagnostic accuracy of PCT, CRP, and WBC from seven studies, which shown that PCT had the highest diagnostic value for complicated appendicitis [18]. In a research made by Kafetzis et al. [19], PCT level >0.5 ng/ml was found to have a predictive value for the diagnosis of appendiceal perforation or gangrene with a sensitivity of 73.4% and a specificity of 94.6% in children. Our results were basically consistent with those in that report.
In the present study, we assessed the diagnostic accuracy of PCT, WBC, and CRP for predicting appendiceal perforation in children. Our study revealed that the preoperative laboratory CRP and PCT parameters are useful for predicting appendiceal perforation in the pediatric patients. The highest sum of sensitivity and specificity for PCT was 0.49ng/mL and a cutoff for PCT of greater than 0.46ng/mL had 93% sensitivity and 92% specificity for appendiceal perforation. Using a cutoff for PCT of greater than 0.5ng/mL, the results of the study made by Kafetzis et al were basically consistent with us with a sensitivity of 73.4% and a specificity of 94.6% [19]. Logistic regression analysis results shown both CRP and PCT are significant markers of a perforated appendicitis. However, the highest sum of sensitivity and specificity for CRP was significant higher than the normal value (55.0 vs 8.0 mg/L).
Our work had several limitations. Firstly, this study is a retrospective study, and surgeons were not blind to the laboratory values before surgery. Secondly, it is hard to get the complete blood test results for each patient, which reduced the number of patients available for our study. Thirdly, we did not analyze the histological results of the non-perforated group and the definition of the stages of appendicitis varies in the literature [20-21]. Lastly, our study had difficulty in identifying the relationship between the blood tests and the subsequent complications such as peritonitis, abscesses, and/or intestinal obstruction.
Our study investigated the diagnostic accuracy of PCT, WBC, and CRP as promising predictive markers for appendiceal perforation in a group of 201 children. We observed that patients with appendiceal perforation have significantly higher PCT and CRP levels than patients without perforation. In addition, specificity and positive predictive value of PCT were considerably higher compared with WBC or CRP. Although logistic regression analysis shown CRP were also associated with perforation with considerable sensitivity and specificity, the highest sum of sensitivity and specificity for it was significant higher than the normal value compared with PCT. Therefore, our study indicated that PCT was an independent predictor of appendiceal perforation and it had the largest area under receiver operating characteristic curve compared with WBC and CRP in predicting appendiceal perforation.
The authors report no conflicts of interest in this work.
This article does not contain any studies with human participants or animals performed by any of the authors.
- Williams RF, Blakely ML, Fischer PE, Streck CJ, Dassinger MS, et al. (2009) Diagnosing ruptured appendicitis preoperatively in pediatric patients. Journal of the American College of surgeons. 208: 819-825. [Crossref]
- Sivit CJ, Siegel MJ, Applegate KE, Newman KD (2001) When appendicitis is suspected in children 1. Radiographics. 21: 247-262. [Crossref]
- Sivit CJ, Newman KD, Boenning DA, Nussbaum-Blask AR, Bulas DI, et al. (1992) Appendicitis: usefulness of US in diagnosis in a pediatric population. Radiology. 185: 549-552. [Crossref]
- Kulik DM, Uleryk EM, Maguire JL (2013) Does this child have appendicitis? A systematic review of clinical prediction rules for children with acute abdominal pain. J Clin Epidemiol. 66: 95-104. [Crossref]
- Singh M, Kadian YS, Rattan KN, Jangra B (2014) Complicated appendicitis: analysis of risk factors in children. Afr J Paediatr Surg. 11: 109-113. [Crossref]
- Aprahamian CJ, Barnhart DC, Bledsoe SE, Vaid Y, Harmon CM (2007) Failure in the nonoperative management of pediatric ruptured appendicitis: predictors and consequences. J Pediatr Surg. 42: 934-938. [Crossref]
- Blomqvist PG, Andersson RE, Granath F, Lambe MP, Ekbom AR (2001) Mortality after appendectomy in sweden,1987–1996. Ann Surg. 233: 455-460. [Crossref]
- Gavela T, Cabeza B, Serrano A, Casado-Flores J (2012) C-reactive protein and procalcitonin are predictors of the severity of acute appendicitis in children. Pediatric emergency care. 28: 416-419. [Crossref]
- Guraya SY, Al-Tuwaijri TA, Khairy GA, Murshid KR (2005) Validity of leukocyte count to predict the severity of acute appendicitis. Saudi medical journal. 26: 1945-1947. [Crossref]
- Henry MC, Gollin G, Islam S, Sylvester K, Walker A (2007) Matched analysis of nonoperative management vs immediate appendectomy for perforated appendicitis. J Pediatr Surg. 42: 19-23. [Crossref]
- Brunkhorst FM, Heinz U, Forycki ZF (1998) Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 24: 888-889. [Crossref]
- Becker KL, Nylen ES, White JC, Müller B, Snider RH Jr (2004) Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. The Journal of Clinical Endocrinology & Metabolism. 89: 1512-1525. [Crossref]
- Assicot M, Bohuon C, Gendrel D, Carsin H, Raymond J, et al. (1993) High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 341: 515-518. [Crossref]
- Benador N, Siegrist CA, Gendrel D, Greder C, Benador D, et al. (1998) Procalcitonin is a marker of severity of renal lesions in pyelonephritis. Pediatrics. 102: 1422-1425. [Crossref]
- Jebali MA, Hausfater P, Abbes Z, Aouni Z, Riou B, et al. (2007) Assessment of the accuracy of procalcitonin to diagnose postoperative infection after cardiac surgery. The Journal of the American Society of Anesthesiologists. 107: 232-238. [Crossref]
- Sand M, Trullen XV, Bechara FG, Pala XF, Sand D, et al. (2009) A prospective bicenter study investigating the diagnostic value of procalcitonin in patients with acute appendicitis. Eur Surg Res. 43: 291-297. [Crossref]
- Kaya B, Sana B, Eris C, Karabulut K, Bat O, et al (2012) The diagnostic value of D-dimer, procalcitonin and CRP in acute appendicitis. Int J Med Sci. 9: 909-915. [Crossref]
- Yu CW, Juan LI, Wu MH, Shen CJ, Wu JY, et al. (2013) Systematic review and meta-analysis of the diagnostic accuracy of procalcitonin, C-reactive protein and white blood cell count for suspected acute appendicitis. Br J Surg. 100: 322-329. [Crossref]
- Kafetzis DA, Velissariou IM, Nikolaides P, Sklavos M, Maktabi M, et al. (2005) Procalcitonin as a predictor of severe appendicitis in children. Eur J Clin Microbiol Infect Dis. 24: 484-487. [Crossref]
- Roberts JK, Behravesh M, Dmitrewski J (2008) Macroscopic findings at appendicectomy are unreliable: implications for laparoscopy and malignant conditions of the appendix. Int J Surg Pathol. 16: 386-390. [Crossref]
- Hussain A, Mahmood H, Singhal T, Balakrishnan S, El-Hasani S (2009) What is positive appendicitis? A new answer to an old question. Clinical, macroscopical and microscopical findings in 200 consecutive appendectomies. Singapore Med J. 50: 1145-1149. [Crossref]

